Endoplasmic reticulum remains continuous and undergoes sheet-to-tubule transformation during cell division in mammalian cells
- PMID: 18056408
- PMCID: PMC2099207
- DOI: 10.1083/jcb.200705112
Endoplasmic reticulum remains continuous and undergoes sheet-to-tubule transformation during cell division in mammalian cells
Abstract
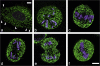
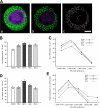
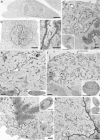
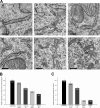
The endoplasmic reticulum (ER) is a multifaceted cellular organelle both structurally and functionally, and its cell cycle-dependent morphological changes are poorly understood. Our quantitative confocal and EM analyses show that the ER undergoes dramatic reorganization during cell division in cultured mammalian cells as mitotic ER profiles become shorter and more branched. 3D modeling by electron tomography reveals that the abundant interphase structures, sheets, are lost and subsequently transform into a branched tubular network that remains continuous. This is confirmed by observing the most prominent ER subdomain, the nuclear envelope (NE). A NE marker protein spreads to the mitotic ER tubules, although it does not show a homogenous distribution within the network. We mimicked the mitotic ER reorganization using puromycin to strip the membrane-bound ribosomes from the interphase ER corresponding to the observed loss of ribosomes normally occurring during mitosis. We propose that the structural changes in mitotic ER are linked to ribosomal action on the ER membranes.
Figures






Similar articles
-
Progressive sheet-to-tubule transformation is a general mechanism for endoplasmic reticulum partitioning in dividing mammalian cells.Mol Biol Cell. 2012 Jul;23(13):2424-32. doi: 10.1091/mbc.E10-12-0950. Epub 2012 May 9. Mol Biol Cell. 2012. PMID: 22573885 Free PMC article.
-
Cisternal organization of the endoplasmic reticulum during mitosis.Mol Biol Cell. 2009 Aug;20(15):3471-80. doi: 10.1091/mbc.e09-04-0327. Epub 2009 Jun 3. Mol Biol Cell. 2009. PMID: 19494040 Free PMC article.
-
Multiple mechanisms determine ER network morphology during the cell cycle in Xenopus egg extracts.J Cell Biol. 2013 Dec 9;203(5):801-14. doi: 10.1083/jcb.201308001. Epub 2013 Dec 2. J Cell Biol. 2013. PMID: 24297752 Free PMC article.
-
Taking organelles apart, putting them back together and creating new ones: lessons from the endoplasmic reticulum.Prog Histochem Cytochem. 2011 Jun;46(1):1-48. doi: 10.1016/j.proghi.2011.04.001. Epub 2011 May 4. Prog Histochem Cytochem. 2011. PMID: 21536318 Review.
-
Sculpting nuclear envelope identity from the endoplasmic reticulum during the cell cycle.Nucleus. 2024 Dec;15(1):2299632. doi: 10.1080/19491034.2023.2299632. Epub 2024 Jan 18. Nucleus. 2024. PMID: 38238284 Free PMC article. Review.
Cited by
-
Rapid intracellular competition between hepatitis C viral genomes as a result of mitosis.J Virol. 2013 Jan;87(1):581-96. doi: 10.1128/JVI.01047-12. Epub 2012 Oct 24. J Virol. 2013. PMID: 23097449 Free PMC article.
-
Spatial reorganization of the endoplasmic reticulum during mitosis relies on mitotic kinase cyclin A in the early Drosophila embryo.PLoS One. 2015 Feb 17;10(2):e0117859. doi: 10.1371/journal.pone.0117859. eCollection 2015. PLoS One. 2015. PMID: 25689737 Free PMC article.
-
Further assembly required: construction and dynamics of the endoplasmic reticulum network.EMBO Rep. 2010 Jul;11(7):515-21. doi: 10.1038/embor.2010.92. Epub 2010 Jun 18. EMBO Rep. 2010. PMID: 20559323 Free PMC article. Review.
-
The endoplasmic reticulum: structure, function and response to cellular signaling.Cell Mol Life Sci. 2016 Jan;73(1):79-94. doi: 10.1007/s00018-015-2052-6. Epub 2015 Oct 3. Cell Mol Life Sci. 2016. PMID: 26433683 Free PMC article. Review.
-
Rapid microwave fixation of cell monolayers preserves microtubule-associated cell structures.J Histochem Cytochem. 2008 Jul;56(7):697-709. doi: 10.1369/jhc.7A7370.2008. Epub 2008 Apr 14. J Histochem Cytochem. 2008. PMID: 18413652 Free PMC article.
References
-
- Baumann, O., and B. Walz. 2001. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int. Rev. Cytol. 205:149–214. - PubMed
-
- Bobinnec, Y., C. Marcaillou, X. Morin, and A. Debec. 2003. Dynamics of the endoplasmic reticulum during early development of Drosophila melanogaster. Cell Motil. Cytoskeleton. 54:217–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

