Megf10 regulates the progression of the satellite cell myogenic program
- PMID: 18056409
- PMCID: PMC2099186
- DOI: 10.1083/jcb.200709083
Megf10 regulates the progression of the satellite cell myogenic program
Abstract
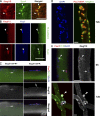
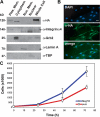
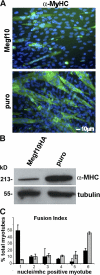
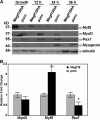
We identify here the multiple epidermal growth factor repeat transmembrane protein Megf10 as a quiescent satellite cell marker that is also expressed in skeletal myoblasts but not in differentiated myofibers. Retroviral expression of Megf10 in myoblasts results in enhanced proliferation and inhibited differentiation. Infected myoblasts that fail to differentiate undergo cell cycle arrest and can reenter the cell cycle upon serum restimulation. Moreover, experimental modulations of Megf10 alter the expression levels of Pax7 and the myogenic regulatory factors. In contrast, Megf10 silencing in activated satellite cells on individual fibers or in cultured myoblasts results in a dramatic reduction in the cell number, caused by myogenin activation and precocious differentiation as well as a depletion of the self-renewing Pax7+/MyoD- population. Additionally, Megf10 silencing in MyoD-/- myoblasts results in down-regulation of Notch signaling components. We conclude that Megf10 represents a novel transmembrane protein that impinges on Notch signaling to regulate the satellite cell population balance between proliferation and differentiation.
Figures



References
-
- Bischoff, R. 1994. The satellite cell and muscle regeneration. In Myogenesis. A.G. Engel and C. Franszini-Armstrong, editors. McGraw-Hill, New York. 97–118.
-
- Braissant, O., and W. Wahli. 1998. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology. 139:2748–2754. - PubMed
-
- Charge, S.B., and M.A. Rudnicki. 2004. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84:209–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

