Runx2 disruption promotes immortalization and confers resistance to oncogene-induced senescence in primary murine fibroblasts
- PMID: 18056452
- PMCID: PMC2562449
- DOI: 10.1158/0008-5472.CAN-07-3016
Runx2 disruption promotes immortalization and confers resistance to oncogene-induced senescence in primary murine fibroblasts
Abstract
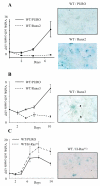
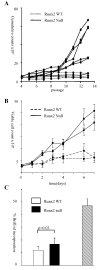
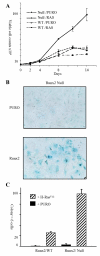
The Runx genes play paradoxical roles in cancer where they can function either as dominant oncogenes or tumor suppressors according to context. We now show that the ability to induce premature senescence in primary murine embryonic fibroblasts (MEF) is a common feature of all three Runx genes. However, ectopic Runx-induced senescence contrasts with Ras oncogene-induced senescence, as it occurs directly and lacks the hallmarks of proliferative stress. Moreover, a fundamental role for Runx function in the senescence program is indicated by the effects of Runx2 disruption, which renders MEFs prone to spontaneous immortalization and confers an early growth advantage that is resistant to stress-induced growth arrest. Runx2(-/-) cells are refractory to H-Ras(V12)-induced premature senescence, despite the activation of a cascade of growth inhibitors and senescence markers, and are permissive for oncogenic transformation. The aberrant behavior of Runx2(-/-) cells is associated with signaling defects and elevated expression of S-G(2)-M cyclins and their associated cyclin dependent kinase activities that may override the effects of growth inhibitory signals. Coupling of stress responses to the cell cycle represents a novel facet of Runx tumor suppressor function and provides a rationale for the lineage-specific effects of loss of Runx function in cancer.
Figures



Similar articles
-
RasV12-mediated down-regulation of CCAAT/enhancer binding protein beta in immortalized fibroblasts requires loss of p19Arf and facilitates bypass of oncogene-induced senescence.Cancer Res. 2009 Mar 15;69(6):2588-98. doi: 10.1158/0008-5472.CAN-08-2312. Epub 2009 Mar 10. Cancer Res. 2009. PMID: 19276382 Free PMC article.
-
p21 loss cooperates with INK4 inactivation facilitating immortalization and Bcl-2-mediated anchorage-independent growth of oncogene-transduced primary mouse fibroblasts.Cancer Res. 2007 May 1;67(9):4130-7. doi: 10.1158/0008-5472.CAN-07-0499. Cancer Res. 2007. PMID: 17483323
-
RUNX1 and its fusion oncoprotein derivative, RUNX1-ETO, induce senescence-like growth arrest independently of replicative stress.Oncogene. 2009 Jul 9;28(27):2502-12. doi: 10.1038/onc.2009.101. Epub 2009 May 18. Oncogene. 2009. PMID: 19448675 Free PMC article.
-
The Runx genes: lineage-specific oncogenes and tumor suppressors.Oncogene. 2004 May 24;23(24):4308-14. doi: 10.1038/sj.onc.1207130. Oncogene. 2004. PMID: 15156187 Review.
-
Functional relationship between p53 and RUNX proteins.J Mol Cell Biol. 2019 Mar 1;11(3):224-230. doi: 10.1093/jmcb/mjy076. J Mol Cell Biol. 2019. PMID: 30535344 Free PMC article. Review.
Cited by
-
The RUNX family: developmental regulators in cancer.Nat Rev Cancer. 2015 Feb;15(2):81-95. doi: 10.1038/nrc3877. Epub 2015 Jan 16. Nat Rev Cancer. 2015. PMID: 25592647 Review.
-
CircRNA-011235 Counteracts The Deleterious Effect of Irradiation Treatment on Bone Mesenchymal Stem Cells by Regulating The miR-741-3p/CDK6 Pathway.Cell J. 2022 Jan;24(1):15-21. doi: 10.22074/cellj.2022.7697. Cell J. 2022. PMID: 35182060 Free PMC article.
-
Genetic deletion of Nrf2 promotes immortalization and decreases life span of murine embryonic fibroblasts.J Gerontol A Biol Sci Med Sci. 2011 Mar;66(3):247-56. doi: 10.1093/gerona/glq181. Epub 2010 Oct 25. J Gerontol A Biol Sci Med Sci. 2011. PMID: 20974733 Free PMC article.
-
The cancer-related Runx2 protein enhances cell growth and responses to androgen and TGFbeta in prostate cancer cells.J Cell Biochem. 2010 Mar 1;109(4):828-37. doi: 10.1002/jcb.22463. J Cell Biochem. 2010. PMID: 20082326 Free PMC article.
-
Cancer-related ectopic expression of the bone-related transcription factor RUNX2 in non-osseous metastatic tumor cells is linked to cell proliferation and motility.Breast Cancer Res. 2010;12(5):R89. doi: 10.1186/bcr2762. Epub 2010 Oct 28. Breast Cancer Res. 2010. PMID: 21029421 Free PMC article.
References
-
- Gergen JP, Butler BA. Isolation of the Drosophila Segmentation Gene Runt and Analysis of Its Expression During Embryogenesis. Genes Dev. 1988;2:1179–93. - PubMed
-
- Blyth K, Cameron ER, Neil JC. The Runx gene family : gain or loss of function in cancer. Nat Rev Cancer. 2005;5:376–87. - PubMed
-
- Wotton S, Stewart M, Blyth K, et al. Proviral insertion indicates a dominant oncogenic role for Runx1/AML1 in T-cell lymphoma. Cancer Res. 2002;62:7181–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

