Protein biomarker identification in the CSF of patients with CNS lymphoma
- PMID: 18056677
- PMCID: PMC4134101
- DOI: 10.1200/JCO.2007.12.1053
Protein biomarker identification in the CSF of patients with CNS lymphoma
Abstract
Purpose: Elucidation of the CSF proteome may yield insights into the pathogenesis of CNS disease. We tested the hypothesis that individual CSF proteins distinguish CNS lymphoma from benign focal brain lesions.
Methods: We used a liquid chromatography/mass spectrometry-based method to differentially quantify and identify several hundred CSF proteins in CNS lymphoma and control patients. We used enzyme-linked immunosorbent assay (ELISA) to confirm one of these markers in an additional validation set of 101 cases.
Results: Approximately 80 CSF proteins were identified and found to be present at significantly different concentrations, both higher and lower, in training and test studies, which were highly concordant. To further validate these observations, we defined in detail the expression of one of these candidate biomarkers, antithrombin III (ATIII). ATIII RNA transcripts were identified within CNS lymphomas, and ATIII protein was localized selectively to tumor neovasculature. Determination of ATIII concentration by ELISA was significantly more accurate (> 75% sensitivity; > 98% specificity) than cytology in the identification of cancer. Measurement of CSF ATIII levels was found to potentially enhance the ability to diagnose and predict outcome.
Conclusion: Our findings demonstrate for the first time that proteomic analysis of CSF yields individual biomarkers with greater sensitivity in the identification of cancer than does CSF cytology. We propose that the discovery of CSF protein biomarkers will facilitate early and noninvasive diagnosis in patients with lesions not amenable to brain biopsy, as well as provide improved surrogates of prognosis and treatment response in CNS lymphoma and brain metastasis.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Figures
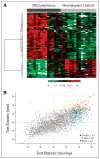






Comment in
-
CSF antithrombin III and disruption of the blood-brain barrier.J Clin Oncol. 2009 May 1;27(13):2302-3; author reply 2303-4. doi: 10.1200/JCO.2008.19.8598. Epub 2009 Mar 30. J Clin Oncol. 2009. PMID: 19332721 No abstract available.
Similar articles
-
Targeted multiplex validation of CSF proteomic biomarkers: implications for differentiation of PCNSL from tumor-free controls and other brain tumors.Front Immunol. 2024 Aug 1;15:1343109. doi: 10.3389/fimmu.2024.1343109. eCollection 2024. Front Immunol. 2024. PMID: 39144147 Free PMC article.
-
A Set of 17 microRNAs Common for Brain and Cerebrospinal Fluid Differentiates Primary Central Nervous System Lymphoma from Non-Malignant Brain Tumors.Biomolecules. 2021 Sep 21;11(9):1395. doi: 10.3390/biom11091395. Biomolecules. 2021. PMID: 34572608 Free PMC article.
-
Elevation of cerebrospinal fluid soluble CD27 levels in patients with meningeal localization of lymphoid malignancies.Blood. 1996 Mar 1;87(5):1985-9. Blood. 1996. PMID: 8634448
-
CSF analysis for protein biomarker identification in patients with leptomeningeal metastases from CNS lymphoma.Expert Rev Proteomics. 2017 Apr;14(4):363-372. doi: 10.1080/14789450.2017.1307106. Expert Rev Proteomics. 2017. PMID: 28293970 Review.
-
Detection of central nervous system involvement in patients with leukemia or non-Hodgkin's lymphoma by immunological marker analysis of cerebrospinal fluid cells.Cancer Treat Res. 1988;38:149-71. doi: 10.1007/978-1-4613-1713-5_3. Cancer Treat Res. 1988. PMID: 2908594 Review. No abstract available.
Cited by
-
Insights into pediatric diffuse intrinsic pontine glioma through proteomic analysis of cerebrospinal fluid.Neuro Oncol. 2012 May;14(5):547-60. doi: 10.1093/neuonc/nos067. Epub 2012 Apr 5. Neuro Oncol. 2012. PMID: 22492959 Free PMC article.
-
Metaprotein expression modeling for label-free quantitative proteomics.BMC Bioinformatics. 2012 May 4;13:74. doi: 10.1186/1471-2105-13-74. BMC Bioinformatics. 2012. PMID: 22559859 Free PMC article.
-
Blood Coagulation Factor Fibrinogen in Tumor Pathogenesis of Central Nervous System B-Cell Lymphoma.Am J Pathol. 2021 Mar;191(3):575-583. doi: 10.1016/j.ajpath.2020.12.010. Am J Pathol. 2021. PMID: 33608067 Free PMC article.
-
Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL).Neuro Oncol. 2012 Mar;14(3):368-80. doi: 10.1093/neuonc/nor203. Epub 2011 Dec 12. Neuro Oncol. 2012. PMID: 22156547 Free PMC article.
-
SILAC-Based Quantitative Proteomic Analysis of Diffuse Large B-Cell Lymphoma Patients.Int J Proteomics. 2015;2015:841769. doi: 10.1155/2015/841769. Epub 2015 Apr 28. Int J Proteomics. 2015. PMID: 26060582 Free PMC article.
References
-
- Josephson SA, Papanastassiou AM, Berger MS, et al. The diagnostic utility of brain biopsy procedures in patients with rapidly deteriorating neurological conditions or dementia. J Neurosurg. 2007;106:72–75. - PubMed
-
- Bernstein M, Berger M. Neuro-Oncology: The Essentials. New York, NY: Thieme Medical Publishers; 2000.
-
- Hormigo A, Abrey L, Heinemann MH, et al. Ocular presentation of primary central nervous system lymphoma: Diagnosis and treatment. Br J Haematol. 2004;126:202–208. - PubMed
-
- Rubenstein JL, Treseler P, O’Brien JM. Pathology and genetics of primary central nervous system and intraocular lymphoma. Hematol Oncol Clin North Am. 2005;19:705–717. - PubMed
-
- Abrey LE, Batchelor TT, Ferrari AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–5043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

