A developmental cycle masks output from the circadian oscillator under conditions of choline deficiency in Neurospora
- PMID: 18056807
- PMCID: PMC2148429
- DOI: 10.1073/pnas.0706631104
A developmental cycle masks output from the circadian oscillator under conditions of choline deficiency in Neurospora
Abstract
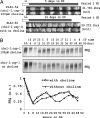
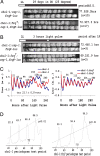
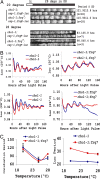
In Neurospora, metabolic oscillators coexist with the circadian transcriptional/translational feedback loop governed by the FRQ (Frequency) and WC (White Collar) proteins. One of these, a choline deficiency oscillator (CDO) observed in chol-1 mutants grown under choline starvation, drives an uncompensated long-period developmental cycle ( approximately 60-120 h). To assess possible contributions of this metabolic oscillator to the circadian system, molecular and physiological rhythms were followed in liquid culture under choline starvation, but these only confirmed that an oscillator with a normal circadian period length can run under choline starvation. This finding suggested that long-period developmental cycles elicited by nutritional stress could be masking output from the circadian system, although a caveat was that the CDO sometimes requires several days to become consolidated. To circumvent this and observe both oscillators simultaneously, we used an assay using a codon-optimized luciferase to follow the circadian oscillator. Under conditions where the long-period, uncompensated, CDO-driven developmental rhythm was expressed for weeks in growth tubes, the luciferase rhythm in the same cultures continued in a typical compensated manner with a circadian period length dependent on the allelic state of frq. Periodograms revealed no influence of the CDO on the circadian oscillator. Instead, the CDO appears as a cryptic metabolic oscillator that can, under appropriate conditions, assume control of growth and development, thereby masking output from the circadian system. frq-driven luciferase as a reporter of the circadian oscillator may in this way provide a means for assessing prospective role(s) of metabolic and/or ancillary oscillators within cellular circadian systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Effects of prd circadian clock mutations on FRQ-less rhythms in Neurospora.J Biol Rhythms. 2010 Apr;25(2):71-80. doi: 10.1177/0748730409360889. J Biol Rhythms. 2010. PMID: 20348458
-
Circadian output, input, and intracellular oscillators: insights into the circadian systems of single cells.Cold Spring Harb Symp Quant Biol. 2007;72:201-14. doi: 10.1101/sqb.2007.72.067. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419278 Free PMC article. Review.
-
Choline depletion, frq mutations, and temperature compensation of the circadian rhythm in Neurospora crassa.J Biol Rhythms. 1998 Aug;13(4):268-77. doi: 10.1177/074873098129000101. J Biol Rhythms. 1998. PMID: 9711502
-
A new mutation affecting FRQ-less rhythms in the circadian system of Neurospora crassa.PLoS Genet. 2011 Jun;7(6):e1002151. doi: 10.1371/journal.pgen.1002151. Epub 2011 Jun 23. PLoS Genet. 2011. PMID: 21731506 Free PMC article.
-
A circadian clock in Neurospora: how genes and proteins cooperate to produce a sustained, entrainable, and compensated biological oscillator with a period of about a day.Cold Spring Harb Symp Quant Biol. 2007;72:57-68. doi: 10.1101/sqb.2007.72.072. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18522516 Free PMC article. Review.
Cited by
-
Habitat-Specific Clock Variation and Its Consequence on Reproductive Fitness.J Biol Rhythms. 2020 Apr;35(2):134-144. doi: 10.1177/0748730419896486. Epub 2019 Dec 26. J Biol Rhythms. 2020. PMID: 31878828 Free PMC article.
-
Circadian rhythms, metabolic oscillators, and the target of rapamycin (TOR) pathway: the Neurospora connection.Curr Genet. 2019 Apr;65(2):339-349. doi: 10.1007/s00294-018-0897-6. Epub 2018 Oct 26. Curr Genet. 2019. PMID: 30367189 Review.
-
period-1 encodes an ATP-dependent RNA helicase that influences nutritional compensation of the Neurospora circadian clock.Proc Natl Acad Sci U S A. 2015 Dec 22;112(51):15707-12. doi: 10.1073/pnas.1521918112. Epub 2015 Dec 8. Proc Natl Acad Sci U S A. 2015. PMID: 26647184 Free PMC article.
-
Making Time: Conservation of Biological Clocks from Fungi to Animals.Microbiol Spectr. 2017 May;5(3):10.1128/microbiolspec.funk-0039-2016. doi: 10.1128/microbiolspec.FUNK-0039-2016. Microbiol Spectr. 2017. PMID: 28527179 Free PMC article. Review.
-
Transcriptional rewiring of an evolutionarily conserved circadian clock.EMBO J. 2024 May;43(10):2015-2034. doi: 10.1038/s44318-024-00088-3. Epub 2024 Apr 16. EMBO J. 2024. PMID: 38627599 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

