A herpesvirus ubiquitin-specific protease is critical for efficient T cell lymphoma formation
- PMID: 18056809
- PMCID: PMC2148416
- DOI: 10.1073/pnas.0706295104
A herpesvirus ubiquitin-specific protease is critical for efficient T cell lymphoma formation
Abstract
The herpesvirus ubiquitin-specific protease (USP) family, whose founding member was discovered as a protease domain embedded in the large tegument protein of herpes simplex virus 1 (HSV-1), is conserved across all members of the Herpesviridae. Whether this conservation is indicative of an essential function of the enzyme in vivo has not yet been established. As reported here, USP activity is conserved in Marek's disease virus (MDV), a tumorigenic alphaherpesvirus. A single amino acid substitution that abolishes the USP activity of the MDV large tegument protein diminishes MDV replication in vivo, and severely limits the oncogenic potential of the virus. Expression of the USP transcripts in MDV-transformed cell lines further substantiates this hypothesis. The herpesvirus USP thus appears to be required not only to maintain a foothold in the immunocompetent host, but also to contribute to malignant outgrowths.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

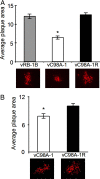
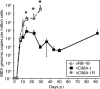


References
-
- Glickman MH, Ciechanover A. Physiol Rev. 2002;82:373–428. - PubMed
-
- Raiborg C, Rusten TE, Stenmark H. Curr Opin Cell Biol. 2003;15:446–455. - PubMed
-
- Sigismund S, Polo S, Di Fiore PP. Curr Top Microbiol Immunol. 2004;286:149–185. - PubMed
-
- Sun LJ, Chen ZJ. Curr Opin Cell Biol. 2004;16:119–126. - PubMed
-
- Weissman AM. Nat Rev Mol Cell Biol. 2001;2:169–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

