Impaired response to GM-CSF and G-CSF, and enhanced apoptosis in C/EBPbeta-deficient hematopoietic cells
- PMID: 18056834
- PMCID: PMC2265449
- DOI: 10.1182/blood-2007-04-087213
Impaired response to GM-CSF and G-CSF, and enhanced apoptosis in C/EBPbeta-deficient hematopoietic cells
Abstract
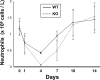
Transcription factors known as CCAAT enhancer binding proteins (C/EBPs) are involved in hematopoietic differentiation, including myelopoiesis and granulopoiesis. C/EBPbeta-deficient mice develop normally; however, they exhibit defective macrophage function, resulting in increased susceptibility to infection. Little is known about the role of C/EBPbeta in granulopoiesis; therefore, we examined granulopoiesis in C/EBPbeta-deficient mice. Morphology, the number of peripheral blood and bone marrow cells, and the expression of genes specific for the myeloid lineage were normal in C/EBPbeta-deficient mice. Interestingly, the hematopoietic progenitor cells of C/EBPbeta-deficient mice did not respond normally to granulocyte/macrophage-colony stimulating factor and granulocyte colony stimulating factor. In addition, C/EBPbeta-deficient neutrophils displayed enhanced apoptosis compared with wild-type neutrophils. Our present results indicate that C/EBPbeta helps regulate survival of neutrophils, downstream of the granulocyte colony stimulating factor receptor.
Figures



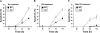
Similar articles
-
Cyclic AMP responsive element binding proteins are involved in 'emergency' granulopoiesis through the upregulation of CCAAT/enhancer binding protein β.PLoS One. 2013;8(1):e54862. doi: 10.1371/journal.pone.0054862. Epub 2013 Jan 30. PLoS One. 2013. PMID: 23382991 Free PMC article.
-
C/EBPbeta is required for 'emergency' granulopoiesis.Nat Immunol. 2006 Jul;7(7):732-9. doi: 10.1038/ni1354. Epub 2006 Jun 4. Nat Immunol. 2006. PMID: 16751774
-
Stimulation of granulopoiesis by transforming growth factor beta: synergy with granulocyte/macrophage-colony-stimulating factor.Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7190-4. doi: 10.1073/pnas.88.16.7190. Proc Natl Acad Sci U S A. 1991. PMID: 1831268 Free PMC article.
-
G-CSF/GM-CSF-induced hematopoietic dysregulation in the progression of solid tumors.FEBS Open Bio. 2022 Jul;12(7):1268-1285. doi: 10.1002/2211-5463.13445. Epub 2022 Jun 9. FEBS Open Bio. 2022. PMID: 35612789 Free PMC article. Review.
-
Effects of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on the bactericidal functions of neutrophils.Curr Opin Hematol. 1997 May;4(3):183-90. doi: 10.1097/00062752-199704030-00005. Curr Opin Hematol. 1997. PMID: 9209834 Review.
Cited by
-
Non-steady-state hematopoiesis regulated by the C/EBPβ transcription factor.Cancer Sci. 2015 Jul;106(7):797-802. doi: 10.1111/cas.12690. Epub 2015 Jun 1. Cancer Sci. 2015. PMID: 25940801 Free PMC article.
-
The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments.Nat Commun. 2021 May 17;12(1):2856. doi: 10.1038/s41467-021-22973-9. Nat Commun. 2021. PMID: 34001893 Free PMC article.
-
Dusp6 deficiency attenuates neutrophil-mediated cardiac damage in the acute inflammatory phase of myocardial infarction.Nat Commun. 2022 Nov 5;13(1):6672. doi: 10.1038/s41467-022-33631-z. Nat Commun. 2022. PMID: 36335128 Free PMC article.
-
Emergency granulopoiesis.Nat Rev Immunol. 2014 May;14(5):302-14. doi: 10.1038/nri3660. Epub 2014 Apr 22. Nat Rev Immunol. 2014. PMID: 24751955 Review.
-
Frontline Science: Myeloid cell-specific deletion of Cebpb decreases sepsis-induced immunosuppression in mice.J Leukoc Biol. 2017 Aug;102(2):191-200. doi: 10.1189/jlb.4HI1216-537R. Epub 2017 May 5. J Leukoc Biol. 2017. PMID: 28476751 Free PMC article.
References
-
- McKnight SL. McBindall: a better name for CCAAT/enhancer binding proteins? Cell. 2001;107:259–261. - PubMed
-
- Friedman AD. Transcriptional regulation of granulocyte and monocyte development. Oncogene. 2002;21:3377–3390. - PubMed
-
- Lekstrom-Himes JA. The role of C/EBP(epsilon) in the terminal stages of granulocyte differentiation. Stem Cells. 2001;19:125–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

