Hematopoiesis and immunity of HOXB4-transduced embryonic stem cell-derived hematopoietic progenitor cells
- PMID: 18056836
- PMCID: PMC2265445
- DOI: 10.1182/blood-2007-10-117366
Hematopoiesis and immunity of HOXB4-transduced embryonic stem cell-derived hematopoietic progenitor cells
Abstract
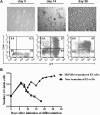
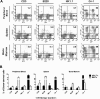
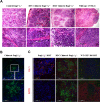
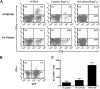
The ability of embryonic stem (ES) cells to form cells and tissues from all 3 germ layers can be exploited to generate cells that can be used to treat diseases. In particular, successful generation of hematopoietic cells from ES cells could provide safer and less immunogenic cells than bone marrow cells, which require severe host preconditioning when transplanted across major histocompatibility complex barriers. Here, we exploited the self-renewal properties of ectopically expressed HOXB4, a homeobox transcription factor, to generate hematopoietic progenitor cells (HPCs) that successfully induce high-level mixed chimerism and long-term engraftment in recipient mice. The HPCs partially restored splenic architecture in Rag2(-/-)gamma(c)(-/-)-immunodeficient mice. In addition, HPC-derived newly generated T cells were able to mount a peptide-specific response to lymphocytic choriomeningitis virus and specifically secreted interleukin-2 and interferon-gamma upon CD3 stimulation. In addition, HPC-derived antigen presenting cells in chimeric mice efficiently presented viral antigen to wild-type T cells. These results demonstrate for the first time that leukocytes derived from ES cells ectopically expressing HOXB4 are immunologically functional, opening up new opportunities for the use of ES cell-derived HPCs in the treatment of hematologic and immunologic diseases.
Figures


Comment in
-
An ES cell-derived immune system.Blood. 2008 Mar 15;111(6):2948-9. doi: 10.1182/blood-2008-01-130369. Blood. 2008. PMID: 23227565 Free PMC article.
Similar articles
-
HOXB4 but not BMP4 confers self-renewal properties to ES-derived hematopoietic progenitor cells.Transplantation. 2008 Dec 27;86(12):1803-9. doi: 10.1097/TP.0b013e31818fe741. Transplantation. 2008. PMID: 19104426 Free PMC article.
-
HOXB4-transduced embryonic stem cell-derived Lin-c-kit+ and Lin-Sca-1+ hematopoietic progenitors express H60 and are targeted by NK cells.J Immunol. 2009 Nov 1;183(9):5449-57. doi: 10.4049/jimmunol.0901807. Epub 2009 Oct 14. J Immunol. 2009. PMID: 19828634
-
HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors.Cell. 2002 Apr 5;109(1):29-37. doi: 10.1016/s0092-8674(02)00680-3. Cell. 2002. PMID: 11955444
-
From embryos to embryoid bodies: generating blood from embryonic stem cells.Ann N Y Acad Sci. 2003 May;996:122-31. doi: 10.1111/j.1749-6632.2003.tb03240.x. Ann N Y Acad Sci. 2003. PMID: 12799290 Review.
-
Control of self-renewal and differentiation of hematopoietic stem cells: HOXB4 on the threshold.Ann N Y Acad Sci. 2005 Jun;1044:6-15. doi: 10.1196/annals.1349.002. Ann N Y Acad Sci. 2005. PMID: 15958692 Review.
Cited by
-
ES-cell derived hematopoietic cells induce transplantation tolerance.PLoS One. 2008 Sep 15;3(9):e3212. doi: 10.1371/journal.pone.0003212. PLoS One. 2008. PMID: 18791641 Free PMC article.
-
Bottlenecks in deriving definitive hematopoietic stem cells from human pluripotent stem cells: a CIRM mini-symposium and workshop report.Stem Cells Transl Med. 2014 Jul;3(7):775-81. doi: 10.5966/sctm.2014-0104. Stem Cells Transl Med. 2014. PMID: 24973044 Free PMC article.
-
Embryonic stem cell-derived haematopoietic progenitor cells down-regulate the CD3 ξ chain on T cells, abrogating alloreactive T cells.Immunology. 2014 Jul;142(3):421-30. doi: 10.1111/imm.12268. Immunology. 2014. PMID: 24527810 Free PMC article.
-
Serum- and stromal cell-free hypoxic generation of embryonic stem cell-derived hematopoietic cells in vitro, capable of multilineage repopulation of immunocompetent mice.Stem Cells Transl Med. 2012 Aug;1(8):581-91. doi: 10.5966/sctm.2012-0020. Epub 2012 Aug 6. Stem Cells Transl Med. 2012. PMID: 23197864 Free PMC article.
-
Experimental limitations using reprogrammed cells for hematopoietic differentiation.J Biomed Biotechnol. 2011;2011:895086. doi: 10.1155/2011/895086. Epub 2011 Nov 30. J Biomed Biotechnol. 2011. PMID: 22187531 Free PMC article. Review.
References
-
- Vodyanik MA, Bork JA, Thomson JA, et al. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. - PubMed
-
- Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. - PubMed
-
- Wang L, Li L, Shojaei F, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. - PubMed
-
- Perlingeiro RC, Kyba M, Daley GQ. Clonal analysis of differentiating embryonic stem cells reveals a hematopoietic progenitor with primitive erythroid and adult lymphoid-myeloid potential. Development. 2001;128:4597–4604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

