Prediction of cytomegalovirus (CMV) plasma load from evaluation of CMV whole-blood load in samples from renal transplant recipients
- PMID: 18057128
- PMCID: PMC2238080
- DOI: 10.1128/JCM.01499-07
Prediction of cytomegalovirus (CMV) plasma load from evaluation of CMV whole-blood load in samples from renal transplant recipients
Abstract
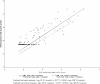
In a prospective cohort of 82 renal transplant recipients, we evaluated the capacity of the cytomegalovirus (CMV) load in whole blood (WB) to predict the plasma CMV load, aiming to identify active CMV infections by using WB samples only and to deduce a WB threshold. Using quantitative real-time PCR, a total of 1,474 WB samples were assayed, of which 279 were positive for CMV, and 140 out of the 276 paired plasma samples tested positive. Thirty (36.6%) patients presented with at least one positive plasma PCR result, and 21 infection episodes (19 patients) required curative treatment (median follow-up time, 12 months). When the plasma CMV load was >500 copies/ml (n = 70), more than 94% (95% confidence interval, 86.0%, 98.4%) of WB samples had >500 copies/ml. Two prediction models were built: log(10) plasma viral load (VL) was calculated as -0.3777 + 0.9342 x log(10) WB VL and as -0.3777 + 0.8563 x log(10) WB VL for patients with and without treatment, respectively. In the validation sample (578 routine samples), 77.2% of the observed and expected plasma viral loads were concordant (95% confidence intervals, 73.5 and 80.5%). According to the model, the plasma viral load was >500 copies/ml when the WB load was >3,170 or >4,000 copies/ml in patients with or without treatment, respectively. WB seems to be an appropriate candidate for routine CMV monitoring of transplant recipients by using a single assay.
Figures
Similar articles
-
Whole blood real-time quantitative PCR for cytomegalovirus infection follow-up in transplant recipients.J Clin Virol. 2006 May;36(1):72-5. doi: 10.1016/j.jcv.2006.01.002. Epub 2006 Feb 14. J Clin Virol. 2006. PMID: 16481215
-
Monitoring of cytomegalovirus viral loads by two molecular assays in whole-blood and plasma samples from hematopoietic stem cell transplant recipients.J Clin Microbiol. 2015 Apr;53(4):1252-7. doi: 10.1128/JCM.03435-14. Epub 2015 Feb 4. J Clin Microbiol. 2015. PMID: 25653404 Free PMC article.
-
Factors influencing cytomegalovirus DNA load measurements in whole blood and plasma specimens from allogeneic hematopoietic stem cell transplant recipients.Diagn Microbiol Infect Dis. 2019 May;94(1):22-27. doi: 10.1016/j.diagmicrobio.2018.11.012. Epub 2018 Nov 22. Diagn Microbiol Infect Dis. 2019. PMID: 30579656
-
Differences in cytomegalovirus plasma viral loads measured in allogeneic hematopoietic stem cell transplant recipients using two commercial real-time PCR assays.J Clin Virol. 2010 Jun;48(2):142-6. doi: 10.1016/j.jcv.2010.03.015. Epub 2010 Apr 14. J Clin Virol. 2010. PMID: 20395168
-
Comparison of two molecular assays for detection of cytomegalovirus DNA in whole blood and plasma samples from transplant recipients.New Microbiol. 2016 Jul;39(3):186-191. Epub 2016 Jun 10. New Microbiol. 2016. PMID: 27284983
Cited by
-
Contribution of Serum Cytomegalovirus PCR to Diagnosis of Early CMV Primary Infection in Pregnant Women.Viruses. 2022 Sep 28;14(10):2137. doi: 10.3390/v14102137. Viruses. 2022. PMID: 36298692 Free PMC article.
-
Primary, Secondary, and Tertiary Prevention of Congenital Cytomegalovirus Infection.Viruses. 2023 Mar 23;15(4):819. doi: 10.3390/v15040819. Viruses. 2023. PMID: 37112800 Free PMC article. Review.
-
Rapid quantitation of cytomegalovirus DNA in whole blood by a new molecular assay based on automated sample preparation and real-time PCR.Med Microbiol Immunol. 2010 Nov;199(4):311-6. doi: 10.1007/s00430-010-0164-z. Epub 2010 Jun 18. Med Microbiol Immunol. 2010. PMID: 20559848
-
Survey of HCMV in allogenic and autologous stem cell transplantation by real-time PCR in Kermanshah, west of Iran.Infect Agent Cancer. 2021 Feb 2;16(1):8. doi: 10.1186/s13027-021-00349-4. Infect Agent Cancer. 2021. PMID: 33531035 Free PMC article.
-
Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation.Clin Microbiol Rev. 2013 Oct;26(4):703-27. doi: 10.1128/CMR.00015-13. Clin Microbiol Rev. 2013. PMID: 24092851 Free PMC article. Review.
References
-
- Barrett-Muir, W., J. Breuer, C. Millar, J. Thomas, D. Jeffries, M. Yaqoob, and C. Aitken. 2000. CMV viral load measurements in whole blood and plasma—which is best following renal transplantation? Transplantation 70116-119. - PubMed
-
- Boeckh, M., G. M. Gallez-Hawkins, D. Myerson, J. A. Zaia, and R. A. Bowden. 1997. Plasma polymerase chain reaction for cytomegalovirus DNA after allogeneic marrow transplantation: comparison with polymerase chain reaction using peripheral blood leukocytes, pp65 antigenemia, and viral culture. Transplantation 64108-113. - PubMed
-
- Boivin, G., R. Belanger, R. Delage, C. Beliveau, C. Demers, N. Goyette, and J. Roy. 2000. Quantitative analysis of cytomegalovirus (CMV) viremia using the pp65 antigenemia assay and the COBAS AMPLICOR CMV MONITOR PCR test after blood and marrow allogeneic transplantation. J. Clin. Microbiol. 384356-4360. - PMC - PubMed
-
- Caliendo, A. M., K. St George, S. Y. Kao, J. Allega, B. H. Tan, R. LaFontaine, L. Bui, and C. R. Rinaldo. 2000. Comparison of quantitative cytomegalovirus (CMV) PCR in plasma and CMV antigenemia assay: clinical utility of the prototype AMPLICOR CMV MONITOR test in transplant recipients. J. Clin. Microbiol. 382122-2127. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

