Prediction of cytomegalovirus (CMV) plasma load from evaluation of CMV whole-blood load in samples from renal transplant recipients
- PMID: 18057128
- PMCID: PMC2238080
- DOI: 10.1128/JCM.01499-07
Prediction of cytomegalovirus (CMV) plasma load from evaluation of CMV whole-blood load in samples from renal transplant recipients
Abstract
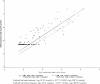
In a prospective cohort of 82 renal transplant recipients, we evaluated the capacity of the cytomegalovirus (CMV) load in whole blood (WB) to predict the plasma CMV load, aiming to identify active CMV infections by using WB samples only and to deduce a WB threshold. Using quantitative real-time PCR, a total of 1,474 WB samples were assayed, of which 279 were positive for CMV, and 140 out of the 276 paired plasma samples tested positive. Thirty (36.6%) patients presented with at least one positive plasma PCR result, and 21 infection episodes (19 patients) required curative treatment (median follow-up time, 12 months). When the plasma CMV load was >500 copies/ml (n = 70), more than 94% (95% confidence interval, 86.0%, 98.4%) of WB samples had >500 copies/ml. Two prediction models were built: log(10) plasma viral load (VL) was calculated as -0.3777 + 0.9342 x log(10) WB VL and as -0.3777 + 0.8563 x log(10) WB VL for patients with and without treatment, respectively. In the validation sample (578 routine samples), 77.2% of the observed and expected plasma viral loads were concordant (95% confidence intervals, 73.5 and 80.5%). According to the model, the plasma viral load was >500 copies/ml when the WB load was >3,170 or >4,000 copies/ml in patients with or without treatment, respectively. WB seems to be an appropriate candidate for routine CMV monitoring of transplant recipients by using a single assay.
Figures
References
-
- Barrett-Muir, W., J. Breuer, C. Millar, J. Thomas, D. Jeffries, M. Yaqoob, and C. Aitken. 2000. CMV viral load measurements in whole blood and plasma—which is best following renal transplantation? Transplantation 70116-119. - PubMed
-
- Boeckh, M., G. M. Gallez-Hawkins, D. Myerson, J. A. Zaia, and R. A. Bowden. 1997. Plasma polymerase chain reaction for cytomegalovirus DNA after allogeneic marrow transplantation: comparison with polymerase chain reaction using peripheral blood leukocytes, pp65 antigenemia, and viral culture. Transplantation 64108-113. - PubMed
-
- Boivin, G., R. Belanger, R. Delage, C. Beliveau, C. Demers, N. Goyette, and J. Roy. 2000. Quantitative analysis of cytomegalovirus (CMV) viremia using the pp65 antigenemia assay and the COBAS AMPLICOR CMV MONITOR PCR test after blood and marrow allogeneic transplantation. J. Clin. Microbiol. 384356-4360. - PMC - PubMed
-
- Caliendo, A. M., K. St George, S. Y. Kao, J. Allega, B. H. Tan, R. LaFontaine, L. Bui, and C. R. Rinaldo. 2000. Comparison of quantitative cytomegalovirus (CMV) PCR in plasma and CMV antigenemia assay: clinical utility of the prototype AMPLICOR CMV MONITOR test in transplant recipients. J. Clin. Microbiol. 382122-2127. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

