A novel, killed-virus nasal vaccinia virus vaccine
- PMID: 18057181
- PMCID: PMC2238057
- DOI: 10.1128/CVI.00440-07
A novel, killed-virus nasal vaccinia virus vaccine
Abstract
Live-virus vaccines for smallpox are effective but have risks that are no longer acceptable for routine use in populations at minimal risk of infection. We have developed a mucosal, killed-vaccinia virus (VV) vaccine based on antimicrobial nanoemulsion (NE) of soybean oil and detergent. Incubation of VV with 10% NE for at least 60 min causes the complete disruption and inactivation of VV. Simple mixtures of NE and VV (Western Reserve serotype) (VV/NE) applied to the nares of mice resulted in both systemic and mucosal anti-VV immunity, virus-neutralizing antibodies, and Th1-biased cellular responses. Nasal vaccination with VV/NE vaccine produced protection against lethal infection equal to vaccination by scarification, with 100% survival after challenge with 77 times the 50% lethal dose of live VV. However, animals protected with VV/NE immunization did after virus challenge have clinical symptoms more extensive than animals vaccinated by scarification. VV/NE-based vaccines are highly immunogenic and induce protective mucosal and systemic immunity without the need for an inflammatory adjuvant or infection with live virus.
Figures
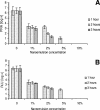

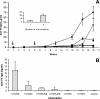
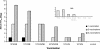
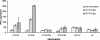


Similar articles
-
Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge.J Virol. 2008 Apr;82(7):3517-29. doi: 10.1128/JVI.01854-07. Epub 2008 Jan 16. J Virol. 2008. PMID: 18199639 Free PMC article.
-
Immune responses to the smallpox vaccine given in combination with ST-246, a small-molecule inhibitor of poxvirus dissemination.Vaccine. 2008 Feb 13;26(7):933-46. doi: 10.1016/j.vaccine.2007.11.095. Epub 2007 Dec 26. Vaccine. 2008. PMID: 18226434 Free PMC article.
-
Protection of mice against the highly pathogenic VVIHD-J by DNA and fowlpox recombinant vaccines, administered by electroporation and intranasal routes, correlates with serum neutralizing activity.Antiviral Res. 2016 Oct;134:182-191. doi: 10.1016/j.antiviral.2016.09.002. Epub 2016 Sep 13. Antiviral Res. 2016. PMID: 27637905 Free PMC article.
-
Third-generation smallpox vaccines: challenges in the absence of clinical smallpox.Future Microbiol. 2010 Sep;5(9):1367-82. doi: 10.2217/fmb.10.98. Future Microbiol. 2010. PMID: 20860482 Review.
-
[Oral and nasal immunization with Poxvirus vacciniae. I. Criteria for smallpox immunity and immunology of the conventional cutaneous reaction to vaccination].Zentralbl Bakteriol Orig B. 1972 Aug;156(1):1-14. Zentralbl Bakteriol Orig B. 1972. PMID: 4575333 Review. German. No abstract available.
Cited by
-
Nanoemulsion nasal adjuvant W₈₀5EC induces dendritic cell engulfment of antigen-primed epithelial cells.Vaccine. 2013 Feb 4;31(7):1072-9. doi: 10.1016/j.vaccine.2012.12.033. Epub 2012 Dec 25. Vaccine. 2013. PMID: 23273511 Free PMC article.
-
Intranasal nanoemulsion-based inactivated respiratory syncytial virus vaccines protect against viral challenge in cotton rats.Hum Vaccin Immunother. 2015;11(12):2904-12. doi: 10.1080/21645515.2015.1075680. Hum Vaccin Immunother. 2015. PMID: 26307915 Free PMC article.
-
Smallpox vaccines for biodefense.Vaccine. 2009 Nov 5;27 Suppl 4:D73-9. doi: 10.1016/j.vaccine.2009.07.103. Vaccine. 2009. PMID: 19837292 Free PMC article. Review.
-
Topological Aspects of the Design of Nanocarriers for Therapeutic Peptides and Proteins.Pharmaceutics. 2019 Feb 21;11(2):91. doi: 10.3390/pharmaceutics11020091. Pharmaceutics. 2019. PMID: 30795556 Free PMC article. Review.
-
Applications of nanotechnology for immunology.Nat Rev Immunol. 2013 Aug;13(8):592-605. doi: 10.1038/nri3488. Nat Rev Immunol. 2013. PMID: 23883969 Free PMC article. Review.
References
-
- Blasco, R., and B. Moss. 1995. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene 158:157-162. - PubMed
-
- Bonilla-Guerrero, R., and G. A. Poland. 2003. Smallpox vaccines: current and future. J. Lab. Clin. Med. 142:252-257. - PubMed
-
- Centers for Disease Control and Prevention. 2003. Multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio and Wisconsin. MMWR Morb. Mortal. Wkly. Rep. 52:642-646. - PubMed
-
- Couch, R. B. 2004. Nasal vaccination, Escherichia coli enterotoxin, and Bell's palsy. N. Engl. J. Med. 350:860-861. - PubMed
-
- Coulibaly, S., P. Bruhl, J. Mayrhofer, K. Schmid, M. Gerencer, and F. G. Falkner. 2005. The nonreplicating smallpox candidate vaccines defective vaccinia Lister (dVV-L) and modified vaccinia Ankara (MVA) elicit robust long-term protection. Virology 341:91-101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

