Activation of hippocampal nuclear factor-kappa B by retrieval is required for memory reconsolidation
- PMID: 18057202
- PMCID: PMC6673108
- DOI: 10.1523/JNEUROSCI.4430-07.2007
Activation of hippocampal nuclear factor-kappa B by retrieval is required for memory reconsolidation
Abstract
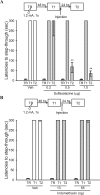
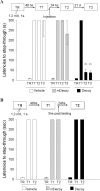
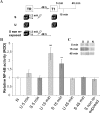
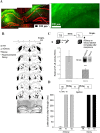
Initially, memory is labile and requires consolidation to become stable. However, several studies support that consolidated memories can undergo a new period of lability after retrieval. The mechanistic differences of this process, termed reconsolidation, with the consolidation process are under debate, including the participation of hippocampus. Up to this point, few reports describe molecular changes and, in particular, transcription factor (TF) involvement in memory restabilization. Increasing evidence supports the participation of the TF nuclear factor-kappaB (NF-kappaB) in memory consolidation. Here, we demonstrate that the inhibition of NF-kappaB after memory reactivation impairs retention of a hippocampal-dependent inhibitory avoidance task in mice. We used two independent disruptive strategies to reach this conclusion. First, we administered intracerebroventricular or intrahippocampal sulfasalazine, an inhibitor of IKK (IkappaB kinase), the kinase that activates NF-kappaB. Second, we infused intracerebroventricular or intrahippocampal kappaB decoy, a direct inhibitor of NF-kappaB consisting of a double-stranded DNA oligonucleotide that contains the kappaB consensus sequence. When injected immediately after memory retrieval, sulfasalazine or kappaB decoy (Decoy) impaired long-term retention. In contrast, a one base mutated kappaB decoy (mDecoy) had no effect. Furthermore, we also found NF-kappaB activation in the hippocampus, with a peak 15 min after memory retrieval. This activation was earlier than that found during consolidation. Together, these results indicate that NF-kappaB is an important transcriptional regulator in memory consolidation and reconsolidation in hippocampus, although the temporal kinetics of activation differs between the two processes.
Figures




References
-
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. - PubMed
-
- Blanchard RJ, Fukunaga KK, Blanchard DC. Environmental control of defensive reaction to footshock. Bull Psychon Soc. 1976;8:129–130.
-
- Boccia MM, Acosta GB, Blake MG, Baratti CM. Memory consolidation and reconsolidation of an inhibitory avoidance response in mice: effects of i.c.v. injections of hemicholinium-3. Neurosci. 2004;124:735–741. - PubMed
-
- Boccia MM, Blake MG, Acosta GB, Baratti CM. Memory consolidation and reconsolidation of an inhibitory avoidance task in mice: effects of a new different learning task. Neuroscience. 2005;135:19–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
