EphB receptors regulate stem/progenitor cell proliferation, migration, and polarity during hippocampal neurogenesis
- PMID: 18057206
- PMCID: PMC6673089
- DOI: 10.1523/JNEUROSCI.4158-07.2007
EphB receptors regulate stem/progenitor cell proliferation, migration, and polarity during hippocampal neurogenesis
Abstract
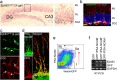
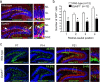
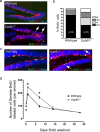
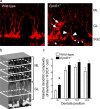
The adult brain maintains two regions of neurogenesis from which new neurons are born, migrate to their appropriate location, and become incorporated into the circuitry of the CNS. One of these, the subgranular zone of the hippocampal dentate gyrus, is of primary interest because of the role of this region in learning and memory. We show that mice lacking EphB1, and more profoundly EphB1 and EphB2, have significantly fewer neural progenitors in the hippocampus. Furthermore, other aspects of neurogenesis, such as polarity, cell positioning, and proliferation are disrupted in animals lacking the EphB1 receptor or its cognate ephrin-B3 ligand. Our data strongly suggest that EphB1 and ephrin-B3 cooperatively regulate the proliferation and migration of neural progenitors in the hippocampus and should be added to a short list of candidate target molecules for modulating the production and integration of new neurons as a treatment for neurodegenerative diseases or brain injury.
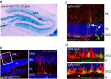
Figures

References
-
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. - PubMed
-
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
-
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. - PubMed
-
- Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Brauninger M, Eilken HM, Rieger MA, Schroeder TT, Huttner WB, Brakebusch C, Gotz M. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–1107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
