Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses
- PMID: 18057210
- PMCID: PMC6673103
- DOI: 10.1523/JNEUROSCI.3151-07.2007
Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses
Abstract
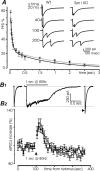
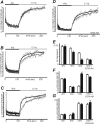
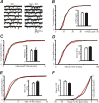
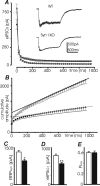
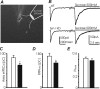
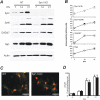
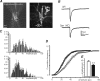
Synapsins (Syns) are synaptic vesicle (SV) phosphoproteins that play a role in neurotransmitter release and synaptic plasticity by acting at multiple steps of exocytosis. Mutation of SYN genes results in an epileptic phenotype in mouse and man suggesting a role of Syns in the control of network excitability. We have studied the effects of the genetic ablation of the SYN1 gene on inhibitory synaptic transmission in primary hippocampal neurons. Inhibitory neurons lacking SynI showed reduced amplitude of IPSCs evoked by isolated action potentials. The impairment in inhibitory transmission was caused by a decrease in the size of the SV readily releasable pool, rather than by changes in release probability or quantal size. The reduction of the readily releasable pool was caused by a decrease in the number of SVs released by single synaptic boutons in response to the action potential, in the absence of variations in the number of synaptic contacts between couples of monosynaptically connected neurons. The deletion of SYN1 did not affect paired-pulse depression or post-tetanic potentiation, but was associated with a moderate increase of synaptic depression evoked by trains of action potentials, which became apparent at high stimulation frequencies and was accompanied by a slow down of recovery from depression. The decreased size of the SV readily releasable pool, coupled with a decreased SV recycling rate and refilling by the SV reserve pool, may contribute to the epileptic phenotype of SynI knock-out mice.
Figures


References
-
- Atwood HL, Karunanithi S. Diversification of synaptic strength: presynaptic elements. Nat Rev Neurosci. 2002;3:497–516. - PubMed
-
- Baldelli P, Forni PE, Carbone E. BDNF, NT-3 and NGF induce distinct new Ca2+ channel synthesis in developing hippocampal neurons. Eur J Neurosci. 2000;12:4017–4032. - PubMed
-
- Baldelli P, Novara M, Carabelli V, Hernández-Guijo JM, Carbone E. BDNF up-regulates evoked GABAergic transmission in developing hippocampal neurons by potentiating presynaptic N- and P/Q-type Ca2+ channels signaling. Eur J Neurosci. 2002;16:2297–2310. - PubMed
-
- Baldelli P, Fassio A, Corradi A, Valtorta F, Benfenati F. The synapsins and the control of neuroexocytosis. In: Regazzi R, editor. Molecular mechanisms of exocytosis. Georgetown, TX: Landes Bioscence; 2006. pp. 62–74.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
