Equine infectious anemia virus entry occurs through clathrin-mediated endocytosis
- PMID: 18057237
- PMCID: PMC2258727
- DOI: 10.1128/JVI.01754-07
Equine infectious anemia virus entry occurs through clathrin-mediated endocytosis
Abstract
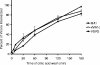
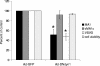
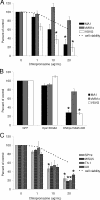
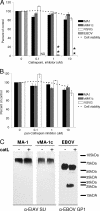
Entry of wild-type lentivirus equine infectious anemia virus (EIAV) into cells requires a low-pH step. This low-pH constraint implicates endocytosis in EIAV entry. To identify the endocytic pathway involved in EIAV entry, we examined the entry requirements for EIAV into two different cells: equine dermal (ED) cells and primary equine endothelial cells. We investigated the entry mechanism of several strains of EIAV and found that both macrophage-tropic and tissue culture-adapted strains utilize clathrin-coated pits for entry. In contrast, a superinfecting strain of EIAV, EIAV(vMA-1c), utilizes two mechanisms of entry. In cells such as ED cells that EIAV(vMA-1c) is able to superinfect, viral entry is pH independent and appears to be mediated by plasma membrane fusion, whereas in cells where no detectable superinfection occurs, EIAV(vMA-1c) entry that is low-pH dependent occurs through clathrin-coated pits in a manner similar to wild-type virus. Regardless of the mechanism of entry being utilized, the internalization kinetics of EIAV is rapid with 50% of cell-associated virions internalizing within 60 to 90 min. Cathepsin inhibitors did not prevent EIAV entry, suggesting that the low-pH step required by wild-type EIAV is not required to activate cellular cathepsins.
Figures
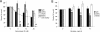
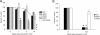

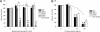
Similar articles
-
Endocytosis and a low-pH step are required for productive entry of equine infectious anemia virus.J Virol. 2005 Dec;79(23):14482-8. doi: 10.1128/JVI.79.23.14482-14488.2005. J Virol. 2005. PMID: 16282447 Free PMC article.
-
An equine infectious anemia virus variant superinfects cells through novel receptor interactions.J Virol. 2008 Oct;82(19):9425-32. doi: 10.1128/JVI.01142-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667522 Free PMC article.
-
Receptor-mediated entry by equine infectious anemia virus utilizes a pH-dependent endocytic pathway.J Virol. 2005 Dec;79(23):14489-97. doi: 10.1128/JVI.79.23.14489-14497.2005. J Virol. 2005. PMID: 16282448 Free PMC article.
-
Characterization of a cytolytic strain of equine infectious anemia virus.J Virol. 2003 Feb;77(4):2385-99. doi: 10.1128/jvi.77.4.2385-2399.2003. J Virol. 2003. PMID: 12551976 Free PMC article.
-
Regulation of equine infectious anemia virus expression.J Biomed Sci. 1998;5(1):11-23. doi: 10.1007/BF02253351. J Biomed Sci. 1998. PMID: 9570509 Review.
Cited by
-
Inhibition of lentivirus replication by aqueous extracts of Prunella vulgaris.Virol J. 2009 Jan 20;6:8. doi: 10.1186/1743-422X-6-8. Virol J. 2009. PMID: 19154592 Free PMC article.
-
HIV-1 entry in SupT1-R5, CEM-ss, and primary CD4+ T cells occurs at the plasma membrane and does not require endocytosis.J Virol. 2014 Dec;88(24):13956-70. doi: 10.1128/JVI.01543-14. Epub 2014 Sep 24. J Virol. 2014. PMID: 25253335 Free PMC article.
-
Host cell factors and functions involved in vesicular stomatitis virus entry.J Virol. 2009 Jan;83(1):440-53. doi: 10.1128/JVI.01864-08. Epub 2008 Oct 29. J Virol. 2009. PMID: 18971266 Free PMC article.
-
Interplay between HIV entry and transportin-SR2 dependency.Retrovirology. 2011 Jan 30;8:7. doi: 10.1186/1742-4690-8-7. Retrovirology. 2011. PMID: 21276267 Free PMC article.
-
Equine Infectious Anemia Virus Cellular Partners Along the Viral Cycle.Viruses. 2024 Dec 24;17(1):5. doi: 10.3390/v17010005. Viruses. 2024. PMID: 39861793 Free PMC article. Review.
References
-
- Barnard, R. J., and J. A. Young. 2003. Alpharetrovirus envelope-receptor interactions. Curr. Top. Microbiol. Immunol. 281107-136. - PubMed
-
- Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 1121303-1311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

