Translocation and colocalization of ICP4 and ICP0 in cells infected with herpes simplex virus 1 mutants lacking glycoprotein E, glycoprotein I, or the virion host shutoff product of the UL41 gene
- PMID: 18057247
- PMCID: PMC2258734
- DOI: 10.1128/JVI.02157-07
Translocation and colocalization of ICP4 and ICP0 in cells infected with herpes simplex virus 1 mutants lacking glycoprotein E, glycoprotein I, or the virion host shutoff product of the UL41 gene
Abstract
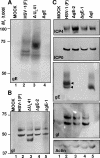
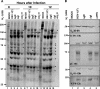
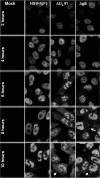
In wild-type herpes simplex virus 1-infected cells, the major regulatory protein ICP4 resides in the nucleus whereas ICP0 becomes dynamically associated with proteasomes and late in infection is translocated and dispersed in the cytoplasm. Inhibition of proteasomal function results in retention or transport of ICP0 to the nucleus. We report that in cells infected with mutants lacking glycoprotein E (gE), glycoprotein I (gI), or the product of the U(L)41 gene, both ICP4 and ICP0 are translocated to the cytoplasm and coaggregate in small dense structures that, in the presence of proteasomal inhibitor MG132, also contain proteasomal components. Gold particle-conjugated antibody to ICP0 reacted in thin sections with dense protein aggregates in the cytoplasm of mutant virus-infected cells. Similar aggregates were present in the nuclei but not in the cytoplasm of wild-type virus-infected cells. Exposure of cells early in infection to MG132 does not result in retention of ICP0 as in wild-type virus-infected cells. The results suggest that the retention of ICP4 and ICP0 in the nucleus is a dynamic process that involves the function of other viral proteins that may include the Fc receptor formed by the gE/gI complex and is not merely the consequence of expression of a nuclear localization signal. It is noteworthy that in DeltaU(L)41-infected cells gE is retained in the trans-Golgi network and is not widely dispersed in cellular membranes.
Figures

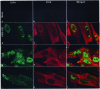
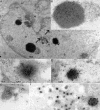
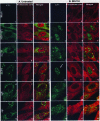
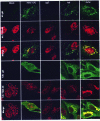
Similar articles
-
Requirements for the nuclear-cytoplasmic translocation of infected-cell protein 0 of herpes simplex virus 1.J Virol. 2001 Apr;75(8):3832-40. doi: 10.1128/JVI.75.8.3832-3840.2001. J Virol. 2001. PMID: 11264372 Free PMC article.
-
Cooperativity among herpes simplex virus type 1 immediate-early regulatory proteins: ICP4 and ICP27 affect the intracellular localization of ICP0.J Virol. 1994 May;68(5):3027-40. doi: 10.1128/JVI.68.5.3027-3040.1994. J Virol. 1994. PMID: 8151771 Free PMC article.
-
Posttranslational processing of infected cell proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize.J Virol. 2001 Sep;75(17):7904-12. doi: 10.1128/jvi.75.17.7904-7912.2001. J Virol. 2001. PMID: 11483735 Free PMC article.
-
Characterization of Elements Regulating the Nuclear-to-Cytoplasmic Translocation of ICP0 in Late Herpes Simplex Virus 1 Infection.J Virol. 2018 Jan 2;92(2):e01673-17. doi: 10.1128/JVI.01673-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093084 Free PMC article.
-
Overexpression of the herpes simplex virus type 1 immediate-early regulatory protein, ICP27, is responsible for the aberrant localization of ICP0 and mutant forms of ICP4 in ICP4 mutant virus-infected cells.J Virol. 1996 Aug;70(8):5346-56. doi: 10.1128/JVI.70.8.5346-5356.1996. J Virol. 1996. PMID: 8764045 Free PMC article.
Cited by
-
Enhanced pathogenesis of an attenuated herpes simplex virus for mice lacking Stat1.J Virol. 2008 Jun;82(12):6052-5. doi: 10.1128/JVI.00297-08. Epub 2008 Apr 9. J Virol. 2008. PMID: 18400863 Free PMC article.
-
Cellular localization of the herpes simplex virus ICP0 protein dictates its ability to block IRF3-mediated innate immune responses.PLoS One. 2010 Apr 29;5(4):e10428. doi: 10.1371/journal.pone.0010428. PLoS One. 2010. PMID: 20454685 Free PMC article.
-
Herpes simplex virus virion host shutoff attenuates establishment of the antiviral state.J Virol. 2008 Jun;82(11):5527-35. doi: 10.1128/JVI.02047-07. Epub 2008 Mar 26. J Virol. 2008. PMID: 18367525 Free PMC article.
-
Biogenesis of Extracellular Vesicles during Herpes Simplex Virus 1 Infection: Role of the CD63 Tetraspanin.J Virol. 2019 Jan 4;93(2):e01850-18. doi: 10.1128/JVI.01850-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30355691 Free PMC article.
-
Nuclear retention of ICP0 in cells exposed to HDAC inhibitor or transfected with DNA before infection with herpes simplex virus 1.Proc Natl Acad Sci U S A. 2008 Dec 23;105(51):20488-93. doi: 10.1073/pnas.0810879105. Epub 2008 Dec 10. Proc Natl Acad Sci U S A. 2008. PMID: 19073918 Free PMC article.
References
-
- Basu, S., G. Dubin, M. Basu, V. Nguyen, and H. M. Friedman. 1995. Characterization of regions of herpes simplex virus type 1 glycoprotein E involved in binding the Fc domain of monomeric IgG and in forming a complex with glycoprotein I. J. Immunol. 154260-267. - PubMed
-
- Basu, S., G. Dubin, T. Nagashunmugam, M. Basu, L. T. Goldstein, L. Wang, B. Weeks, and H. M. Friedman. 1997. Mapping regions of herpes simplex virus type 1 glycoprotein I required for formation of the viral Fc receptor for monomeric IgG. J. Immunol. 158209-215. - PubMed
-
- Brideau, A. D., L. W. Enquist, and R. S. Tirabassi. 2000. The role of virion membrane protein endocytosis in the herpesvirus life cycle. J. Clin. Virol. 1769-82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

