Analyses of the role of endogenous SPARC in mouse models of prostate and breast cancer
- PMID: 18058030
- PMCID: PMC3252392
- DOI: 10.1007/s10585-007-9126-2
Analyses of the role of endogenous SPARC in mouse models of prostate and breast cancer
Abstract
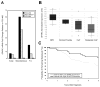
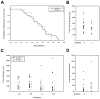
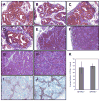
Secreted protein, acidic and rich in cysteine (SPARC, also known as osteonectin or BM-40) is a glycoprotein component of the extracellular matrix that has been reported to be involved with a variety of cellular processes. Although SPARC expression levels are frequently altered in a variety of tumor types, the exact implications of deregulated SPARC expression--whether it promotes, inhibits or has no effect on tumor progression--have remained unclear. Our recent gene expression analyses have shown that SPARC is significantly downregulated in highly metastatic human prostate cancer cells. To test the role of endogenous SPARC in tumorigenesis directly, we examined cancer progression and metastasis in SPARC(+/-) and SPARC(-/-) mice using two separate transgenic mouse tumor models: transgenic adenocarcinoma of the mouse prostate (TRAMP) and murine mammary tumor virus-polyoma middle T (MMTV-PyMT). Surprisingly, in both instances, we found that loss of SPARC had no significant effects on tumor initiation, progression or metastasis. Tumor angiogenesis and collagen deposition were also largely unaffected. Our results indicate that, although differential SPARC expression may be a useful marker of aggressive, metastasis-prone tumors, loss of SPARC is not sufficient either to promote or to inhibit cancer progression in two spontaneous mouse tumor models.
Figures


References
-
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. - PubMed
-
- Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? J Cell Biochem. 2004;92:679–690. - PubMed
-
- Bradshaw AD, Reed MJ, Sage EH. SPARC-null mice exhibit accelerated cutaneous wound closure. J Histochem Cytochem. 2002;50:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

