A phase I safety, pharmacological, and biological study of the farnesyl protein transferase inhibitor, lonafarnib (SCH 663366), in combination with cisplatin and gemcitabine in patients with advanced solid tumors
- PMID: 18058098
- PMCID: PMC2813768
- DOI: 10.1007/s00280-007-0646-x
A phase I safety, pharmacological, and biological study of the farnesyl protein transferase inhibitor, lonafarnib (SCH 663366), in combination with cisplatin and gemcitabine in patients with advanced solid tumors
Abstract
Purpose: This phase I study was conducted to evaluate the safety, tolerability, pharmacological properties and biological activity of the combination of the lonafarnib, a farnesylproteintransferase (FTPase) inhibitor, with gemcitabine and cisplatin in patients with advanced solid malignancies.
Experimental design: This was a single institution study to determine the maximal tolerated dose (MTD) of escalating lonafarnib (75-125 mg po BID) with gemcitabine (750-1,000 mg/m(2) on days 1, 8, 15) and fixed cisplatin (75 mg/m(2) day 1) every 28 days. Due to dose-limiting toxicities (DLTs) of neutropenia and thrombocytopenia in initial patients, these patients were considered "heavily pre-treated" and the protocol was amended to limit prior therapy and re-escalate lonafarnib in "less heavily pre-treated patients" on 28-day and 21-day schedules. Cycle 1 and 2 pharmacokinetics (PK), and farnesylation of the HDJ2 chaperone protein and FPTase activity were analyzed.
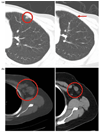
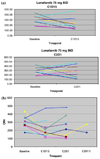
Results: Twenty-two patients received 53 courses of therapy. Nausea, vomiting, and fatigue were frequent in all patients. Severe toxicities were observed in 91% of patients: neutropenia (41%), nausea (36%), thrombocytopenia (32%), anemia (23%) and vomiting (23%). Nine patients withdrew from the study due to toxicity. DLTs of neutropenia, febrile neutropenia, thrombocytopenia, and fatigue limited dose-escalation on the 28-day schedule. The MTD was established as lonafarnib 75 mg BID, gemcitabine 750 mg/m(2) days 1, 8, 15, and cisplatin 75 mg/m(2) in heavily pre-treated patients. The MTD in the less heavily pre-treated patients could not be established on the 28-day schedule as DLTs were observed at the lowest dose level, and dose escalation was not completed on the 21-day schedule due to early study termination by the Sponsor. No PK interactions were observed. FTPase inhibition was not observed at the MTD, however HDJ-2 gel shift was observed in one patient at the 100 mg BID lonafarnib dose. Anti-cancer activity was observed: four patients had stable disease lasting >2 cycles, one subject had a complete response, and another had a partial response, both with metastatic breast cancer.
Conclusion: Lonafarnib 75 mg BID, gemcitabine 750 mg/m(2) days 1, 8, 15, and cisplatin 75 mg/m(2) day 1 on a 28-day schedule was established as the MTD. Lonafarnib did not demonstrate FTPase inhibition at these doses. Despite the observed efficacy, substantial toxicity and questionable contribution of anti-tumor activity of lonafarnib to gemcitabine and cisplatin limits further exploration of this combination.
Figures


References
-
- Adamo V, Magno C, Spitaleri G, Garipoli C, Maisano C, Alafaci E, Adamo B, Rossello R, Scandurra G, Scimone A. Phase II study of gemcitabine and cisplatin in patients with advanced or metastatic bladder cancer: long-term follow-up of a 3-week regimen. Oncology. 2005;69:391–398. - PubMed
-
- Adjei AA, Croghan GA, Erlichman C, Marks RS, Reid JM, Sloan JA, Pitot HC, Alberts SR, Goldberg RM, Hanson LJ, Bruzek LM, Atherton P, Thibault A, Palmer PA, Kaufmann SH. A Phase I trial of the farnesyl protein transferase inhibitor R115777 in combination with gemcitabine and cisplatin in patients with advanced cancer. Clin Cancer Res. 2003;9:2520–2526. - PubMed
-
- Adjei AA, Davis JN, Bruzek LM, Erlichman C, Kaufmann SH. Synergy of the protein farnesyltransferase inhibitor SCH66336 and cisplatin in human cancer cell lines. Clin Cancer Res. 2001;7:1438–1445. - PubMed
-
- Adjei AA, Davis JN, Erlichman C, Svingen PA, Kaufmann SH. Comparison of potential markers of farnesyltransferase inhibition. Clin Cancer Res. 2000;6:2318–2325. - PubMed
-
- Adjei AA, Erlichman C, Davis JN, Cutler DL, Sloan JA, Marks RS, Hanson LJ, Svingen PA, Atherton P, Bishop WR, Kirschmeier P, Kaufmann SH. A Phase I trial of the farnesyl transferase inhibitor SCH66336: evidence for biological and clinical activity. Cancer Res. 2000;60:1871–1877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

