The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2
- PMID: 18058195
- PMCID: PMC2254553
- DOI: 10.1007/s10555-007-9105-8
The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2
Abstract
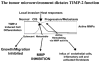
Proteolytic remodeling of the extracellular matrix is an important component of disease progression in many chronic disease states and is the initiating event in the formation of the tumor microenvironment in cancer. It is the balance of extracellular matrix degrading enzymes, the matrix metalloproteinases (MMPs) and their endogenous inhibitors that determine the extent of tissue remodeling. Unchecked MMP activity can result in significant tissue damage, facilitate disease progression and is associated with host responses to pathologic injury such as angiogenesis and inflammation. The tissue inhibitors of metalloproteinases (TIMPs) have been shown to regulate MMP activity. However, recent findings demonstrate that the tissue inhibitor of metalloproteinases-2 (TIMP-2) inhibits the mitogenic response of human microvascular endothelial cells to growth factors, such as VEGF-A and FGF-2 in vitro and angiogenesis in vivo. The mechanism of this effect is independent of metalloproteinase inhibition. Our lab is the first to demonstrate a cell-surface signaling receptor for a member of the TIMP family and suggest that TIMP-2 functions to regulate cellular responses to growth factors. These new findings are discussed in terms of a model of TIMP-2 regulation of cellular functions in the tumor microenvironment.
Figures

Similar articles
-
TIMP-2: an endogenous inhibitor of angiogenesis.Trends Mol Med. 2005 Mar;11(3):97-103. doi: 10.1016/j.molmed.2005.01.007. Trends Mol Med. 2005. PMID: 15760767 Review.
-
Matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases and angiogenic cytokines in peripheral blood of patients with thyroid cancer.Thyroid. 2002 Aug;12(8):655-62. doi: 10.1089/105072502760258622. Thyroid. 2002. PMID: 12225633
-
TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism.Cell. 2003 Jul 25;114(2):171-80. doi: 10.1016/s0092-8674(03)00551-8. Cell. 2003. PMID: 12887919
-
Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro.Exp Mol Med. 2001 Jun 30;33(2):95-102. doi: 10.1038/emm.2001.17. Exp Mol Med. 2001. PMID: 11460888
-
An alternate perspective on the roles of TIMPs and MMPs in pathology.Am J Pathol. 2012 Jan;180(1):12-6. doi: 10.1016/j.ajpath.2011.09.008. Epub 2011 Oct 25. Am J Pathol. 2012. PMID: 22033229 Review.
Cited by
-
Matrix metalloproteinase 9 is a distal-less 3 target-gene in placental trophoblast cells.Am J Physiol Cell Physiol. 2013 Jul 15;305(2):C173-81. doi: 10.1152/ajpcell.00205.2012. Epub 2013 May 8. Am J Physiol Cell Physiol. 2013. PMID: 23657566 Free PMC article.
-
Matrix metalloproteinases and tissue inhibitor of metalloproteinases are essential for the inflammatory response in cancer cells.J Signal Transduct. 2010;2010:985132. doi: 10.1155/2010/985132. Epub 2010 Jul 20. J Signal Transduct. 2010. PMID: 21152266 Free PMC article.
-
Association of MMP2 and MMP9 gene polymorphisms with nonsyndromic cleft lip/palate in an Iranian population.J Dent Res Dent Clin Dent Prospects. 2023 Summer;17(3):149-153. doi: 10.34172/joddd.2023.40640. Epub 2023 Nov 11. J Dent Res Dent Clin Dent Prospects. 2023. PMID: 38023796 Free PMC article.
-
The carboxyl terminal trimer of procollagen I induces pro-metastatic changes and vascularization in breast cancer cells xenografts.BMC Cancer. 2009 Feb 18;9:59. doi: 10.1186/1471-2407-9-59. BMC Cancer. 2009. PMID: 19226458 Free PMC article.
-
Metzincin proteases and their inhibitors: foes or friends in nervous system physiology?J Neurosci. 2010 Nov 17;30(46):15337-57. doi: 10.1523/JNEUROSCI.3467-10.2010. J Neurosci. 2010. PMID: 21084591 Free PMC article. Review.
References
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. - PubMed
-
- Folkman J, Hanahan D. Switch to the angiogenic phenotype during tumorigenesis. Princess Takamatsu Symposia. 1991;22:339–347. - PubMed
-
- Heissig B, Hattori K, Friedrich M, Rafii S, Werb Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Current Opinion in Hematology. 2003;10:136–141. - PubMed
-
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science. 2002;295:2387–2392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

