A double blind, placebo-controlled pilot study of galantamine augmentation of antidepressant treatment in older adults with major depression
- PMID: 18058832
- PMCID: PMC4407281
- DOI: 10.1002/gps.1951
A double blind, placebo-controlled pilot study of galantamine augmentation of antidepressant treatment in older adults with major depression
Abstract
Objective: Depression in older adults is often associated with cognitive abnormalities and may predict later development of a primary cognitive disorder. This double-blind, randomized, placebo-controlled pilot study was designed to assess the safety and efficacy of galantamine augmentation of antidepressant treatment for depressive and cognitive symptoms in older adults with major depression.
Methods: Thirty-eight, non-demented older adults (age >50) with major depression were randomized to receive galantamine or placebo augmentation of standard antidepressant pharmacotherapy (venlafaxine XR or citalopram). Mood and cognitive status were monitored for 24 weeks using the 24-item Hamilton Rating Scale for Depression and the Repeatable Battery for the Assessment of Neuropsychological Status.
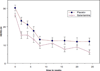
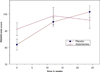
Results: Both groups showed significant improvements in mood and cognition over 24 weeks, but no significant difference was found in change over time between groups. An exploratory post-hoc analysis suggested that patients randomized to galantamine had lower depression scores compared to patients in the placebo group after 2 weeks of treatment. Dropout was high with more subjects randomized to antidepressant plus galantamine withdrawing early from the study.
Conclusions: This pilot study failed to demonstrate a benefit for galantamine augmentation of antidepressant medication in the treatment of depression in older adults. Future studies should explore strategies for reducing dropout in such longitudinal trials and more carefully assess time to response with cholinesterase inhibitor augmentation.
Conflict of interest statement
CONFLICT OF INTEREST
No other affiliations or disclosures are reported for any of the other authors.
Figures
References
-
- Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with ‘reversible dementia’: a controlled study. Am J Psychiatry. 1993;150:1693–1699. - PubMed
-
- Burt T. Donepezil and related cholinesterase inhibitors as mood and behavioral controlling agents. Curr Psychiatry Rep. 2000;2:473–478. - PubMed
-
- Cummings JL, McRae T, Zhang R. Effects of donepezil on neuropsychiatric symptoms in patients with dementia and severe behavioral disorders. Am J Geriatr Psychiatry. 2006;14:605–612. - PubMed
-
- Eden Evins A, Demopulos C, Nierenberg A, et al. A double-blind, placebo-controlled trial of adjunctive donepezil in treatment-resistant mania. Bipolar Disord. 2006;8:75–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical