Effect of oxidatively damaged DNA on the active site preorganization during nucleotide incorporation in a high fidelity polymerase from Bacillus stearothermophilus
- PMID: 18058909
- PMCID: PMC3023110
- DOI: 10.1002/prot.21824
Effect of oxidatively damaged DNA on the active site preorganization during nucleotide incorporation in a high fidelity polymerase from Bacillus stearothermophilus
Abstract
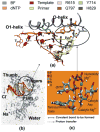
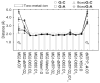
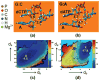
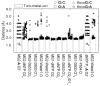
We study the effect of the oxidative lesion 8-oxoguanine (8oxoG) on the preorganization of the active site for DNA replication in the closed (active) state of the Bacillus fragment (BF), a Klenow analog from Bacillus stearothermophilus. Our molecular dynamics and free energy simulations of explicitly solvated model ternary complexes of BF bound to correct dCTP/incorrect dATP opposite guanine (G) and 8oxoG bases in DNA suggest that the lesion introduces structural and energetic changes at the catalytic site to favor dATP insertion. Despite the formation of a stable Watson-Crick pairing in the 8oxoG:dCTP system, the catalytic geometry is severely distorted to possibly slow down catalysis. Indeed, our calculated free energy landscapes associated with active site preorganization suggest additional barriers to assemble an efficient catalytic site, which need to be overcome during dCTP incorporation opposite 8oxoG relative to that opposite undamaged G. In contrast, the catalytic geometry for the Hoogsteen pairing in the 8oxoG:dATP system is highly organized and poised for efficient nucleotide incorporation via the "two-metal-ion" catalyzed phosphoryl transfer mechanism. However, the free energy calculations suggest that the catalytic geometry during dATP incorporation opposite 8oxoG is considerably less plastic than that during dCTP incorporation opposite G despite a very similar, well organized catalytic site for both systems. A correlation analysis of the dynamics trajectories suggests the presence of significant coupling between motions of the polymerase fingers and the primary distance for nucleophilic attack (i.e., between the terminal primer O3' and the dNTP P(alpha.) atoms) during correct dCTP incorporation opposite undamaged G. This coupling is shown to be disrupted during nucleotide incorporation by the polymerase with oxidatively damaged DNA/dNTP substrates. We also suggest that the lesion affects DNA interactions with key polymerase residues, thereby affecting the enzymes ability to discriminate against non-complementary DNA/dNTP substrates. Taken together, our results provide a unified structural, energetic, and dynamic platform to rationalize experimentally observed relative nucleotide incorporation rates for correct dCTP/incorrect dATP insertion opposite an undamaged/oxidatively damaged template G by BF.
2007 Wiley-Liss, Inc.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

