Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels
- PMID: 18059270
- PMCID: PMC2377025
- DOI: 10.1038/ni1550
Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels
Abstract
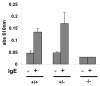
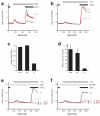
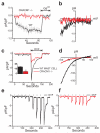
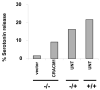
CRACM1 (also called Orai1) constitutes the pore subunit of store-operated calcium release-activated calcium channels. A point mutation in the gene encoding CRACM1 is associated with severe combined immunodeficiency disease in humans. Here we generated CRACM1-deficient mice in which beta-galactosidase activity 'reported' CRACM1 expression. CRACM1-deficient mice were smaller in size. Mast cells derived from CRACM1-deficient mice showed grossly defective degranulation and cytokine secretion, and the allergic reactions elicited in vivo were inhibited in CRACM1-deficient mice. We detected robust CRACM1 expression in skeletal muscles and some regions of the brain, heart and kidney but not in the lymphoid regions of thymus and spleen. In contrast, we found CRACM2 expression to be much higher in mouse T cells. In agreement with those findings, the store-operated calcium influx and development and proliferation of CRACM1-deficient T cells was unaffected. Thus, CRACM1 is crucial in mouse mast cell effector function, but mouse T cell calcium release-activated calcium channels are functional in the absence of CRACM1.
Figures



References
-
- Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

