Functional reactivity of cerebral capillaries
- PMID: 18059431
- PMCID: PMC3197804
- DOI: 10.1038/sj.jcbfm.9600590
Functional reactivity of cerebral capillaries
Abstract
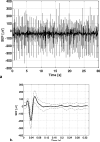
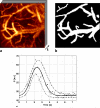
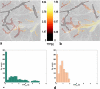
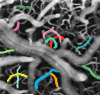
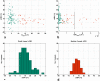
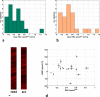
The spatiotemporal evolution of cerebral microcirculatory adjustments to functional brain stimulation is the fundamental determinant of the functional specificity of hemodynamically weighted neuroimaging signals. Very little data, however, exist on the functional reactivity of capillaries, the vessels most proximal to the activated neuronal population. Here, we used two-photon laser scanning microscopy, in combination with intracranial electrophysiology and intravital video microscopy, to explore the changes in cortical hemodynamics, at the level of individual capillaries, in response to steady-state forepaw stimulation in an anesthetized rodent model. Overall, the microcirculatory response to functional stimulation was characterized by a pronounced decrease in vascular transit times (20%+/-8%), a dilatation of the capillary bed (10.9%+/-1.2%), and significant increases in red blood cell speed (33.0%+/-7.7%) and flux (19.5%+/-6.2%). Capillaries dilated more than the medium-caliber vessels, indicating a decreased heterogeneity in vessel volumes and increased blood flow-carrying capacity during neuronal activation relative to baseline. Capillary dilatation accounted for an estimated approximately 18% of the total change in the focal cerebral blood volume. In support of a capacity for focal redistribution of microvascular flow and volume, significant, though less frequent, local stimulation-induced decreases in capillary volume and erythrocyte speed and flux also occurred. The present findings provide further evidence of a strong functional reactivity of cerebral capillaries and underscore the importance of changes in the capillary geometry in the hemodynamic response to neuronal activation.
Figures

References
-
- Ances BM, Greenberg JH, Detre JA. The effects of graded hypercapnia on the activation flow coupling response due to forepaw stimulation in alpha-chloralose anesthetized rats. Brain Res. 2001;911:82–88. - PubMed
-
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992;68:1345–1358. - PubMed
-
- Atkinson JL, Anderson RE, Sundt TM., Jr The effect of carbon dioxide on the diameter of brain capillaries. Brain Res. 1990;517:333–340. - PubMed
-
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. - PubMed
-
- Berwick J, Johnston D, Jones M, Martindale J, Redgrave P, McLoughlin N, Schiessl I, Mayhew JE. Neurovascular coupling investigated with two-dimensional optical imaging spectroscopy in rat whisker barrel cortex. Eur J Neurosci. 2005;22:1655–1666. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

