Neural pathways underlying lactate-induced panic
- PMID: 18059441
- PMCID: PMC3065200
- DOI: 10.1038/sj.npp.1301621
Neural pathways underlying lactate-induced panic
Abstract
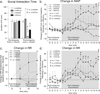
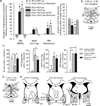
Panic disorder is a severe anxiety disorder characterized by susceptibility to induction of panic attacks by subthreshold interoceptive stimuli such as 0.5 M sodium lactate infusions. Although studied for four decades, the mechanism of lactate sensitivity in panic disorder has not been understood. The dorsomedial hypothalamus/perifornical region (DMH/PeF) coordinates rapid mobilization of behavioral, autonomic, respiratory and endocrine responses to stress, and rats with disrupted GABA inhibition in the DMH/PeF exhibit panic-like responses to lactate, similar to panic disorder patients. Utilizing a variety of anatomical and pharmacological methods, we provide evidence that lactate, via osmosensitive periventricular pathways, activates neurons in the compromised DMH/PeF, which relays this signal to forebrain limbic structures such as the bed nucleus of the stria terminalis to mediate anxiety responses, and specific brainstem sympathetic and parasympathetic pathways to mediate the respiratory and cardiovascular components of the panic-like response. Acutely restoring local GABAergic tone in the DMH/PeF blocked lactate-induced panic-like responses. Autonomic panic-like responses appear to be a result of DMH/PeF-mediated mobilization of sympathetic responses (verified with atenolol) and resetting of the parasympathetically mediated baroreflex. Based on our findings, DMH/PeF efferent targets such as the C1 adrenergic neurons, paraventricular hypothalamus, and the central amygdala are implicated in sympathetic mobilization; the nucleus of the solitary tract is implicated in baroreflex resetting; and the parabrachial nucleus is implicated in respiratory responses. These results elucidate neural circuits underlying lactate-induced panic-like responses and the involvement of both sympathetic and parasympathetic systems.
Figures



References
-
- Abshire VM, Hankins KD, Roehr KE, DiMicco JA. Injection of L-allylglycine into the posterior hypothalamus in rats causes decreases in local GABA which correlate with increases in heart rate. Neuropharmacology. 1988;27:1171–1177. - PubMed
-
- Catelli JM, Sved AF. Enhanced pressor response to GABA in the nucleus tractus solitarii of the spontaneously hypertensive rat. Eur J Pharmacol. 1988;151:243–248. - PubMed
-
- Chen T, Hui R, Dong YX, Li YQ, Mizuno N. Endomorphin 1- and endomorphin 2-like immunoreactive neurons in the hypothalamus send axons to the parabrachial nucleus in the rat. Neurosci Lett. 2004;357:139–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

