Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance
- PMID: 18059474
- PMCID: PMC2206132
- DOI: 10.1038/sj.emboj.7601950
Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance
Abstract
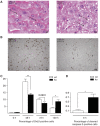
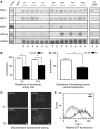
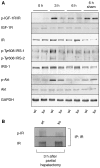
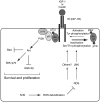
The liver is frequently challenged by surgery-induced metabolic overload, viruses or toxins, which induce the formation of reactive oxygen species. To determine the effect of oxidative stress on liver regeneration and to identify the underlying signaling pathways, we studied liver repair in mice lacking the Nrf2 transcription factor. In these animals, expression of several cytoprotective enzymes was reduced in hepatocytes, resulting in oxidative stress. After partial hepatectomy, liver regeneration was significantly delayed. Using in vitro and in vivo studies, we identified oxidative stress-mediated insulin/insulin-like growth factor resistance as an underlying mechanism. This deficiency impaired the activation of p38 mitogen-activated kinase, Akt kinase and downstream targets after hepatectomy, resulting in enhanced death and delayed proliferation of hepatocytes. Our results reveal novel roles of Nrf2 in the regulation of growth factor signaling and in tissue repair. In addition, they provide new insight into the mechanisms underlying oxidative stress-induced defects in liver regeneration. These findings may provide the basis for the development of new strategies to improve regeneration in patients with acute or chronic liver damage.
Figures




Similar articles
-
The cytoprotective Nrf2 transcription factor controls insulin receptor signaling in the regenerating liver.Cell Cycle. 2008 Apr 1;7(7):874-8. doi: 10.4161/cc.7.7.5617. Epub 2008 Jan 18. Cell Cycle. 2008. PMID: 18414027 Review.
-
Nrf2 activates augmenter of liver regeneration (ALR) via antioxidant response element and links oxidative stress to liver regeneration.Mol Med. 2013 Aug 28;19(1):237-44. doi: 10.2119/molmed.2013.00027. Mol Med. 2013. PMID: 23887691 Free PMC article.
-
Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation.Free Radic Biol Med. 2013 Dec;65:789-799. doi: 10.1016/j.freeradbiomed.2013.08.005. Epub 2013 Aug 14. Free Radic Biol Med. 2013. PMID: 23954472
-
Nrf2 is essential for timely M phase entry of replicating hepatocytes during liver regeneration.Am J Physiol Gastrointest Liver Physiol. 2015 Feb 15;308(4):G262-8. doi: 10.1152/ajpgi.00332.2014. Epub 2014 Dec 18. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25524062 Free PMC article.
-
Insulin signaling and life span.Pflugers Arch. 2010 Jan;459(2):301-14. doi: 10.1007/s00424-009-0721-8. Epub 2009 Sep 13. Pflugers Arch. 2010. PMID: 19756720 Review.
Cited by
-
HCV and HCC Tango-Deciphering the Intricate Dance of Disease: A Review Article.Int J Mol Sci. 2023 Nov 7;24(22):16048. doi: 10.3390/ijms242216048. Int J Mol Sci. 2023. PMID: 38003240 Free PMC article. Review.
-
Phellinus linteus polysaccharides mediates acetaminophen-induced hepatotoxicity via activating AMPK/Nrf2 signaling pathways.Aging (Albany NY). 2022 Sep 1;14(17):6993-7002. doi: 10.18632/aging.204260. Epub 2022 Sep 1. Aging (Albany NY). 2022. PMID: 36057264 Free PMC article.
-
Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo.Diabetes. 2011 Feb;60(2):625-33. doi: 10.2337/db10-1164. Diabetes. 2011. PMID: 21270272 Free PMC article.
-
A low glycemic diet protects disease-prone Nrf2-deficient mice against age-related macular degeneration.Free Radic Biol Med. 2020 Apr;150:75-86. doi: 10.1016/j.freeradbiomed.2020.02.010. Epub 2020 Feb 14. Free Radic Biol Med. 2020. PMID: 32068111 Free PMC article.
-
The Transcription Factor Nrf2 Protects Angiogenic Capacity of Endothelial Colony-Forming Cells in High-Oxygen Radical Stress Conditions.Stem Cells Int. 2017;2017:4680612. doi: 10.1155/2017/4680612. Epub 2017 May 15. Stem Cells Int. 2017. PMID: 28607561 Free PMC article.
References
-
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF (2002) Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277: 1531–1537 - PubMed
-
- Bloch-Damti A, Potashnik R, Gual P, Le Marchand-Brustel Y, Tanti JF, Rudich A, Bashan N (2006) Differential effects of IRS1 phosphorylated on Ser307 or Ser632 in the induction of insulin resistance by oxidative stress. Diabetologia 49: 2463–2473 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

