Cdc2p controls the forkhead transcription factor Fkh2p by phosphorylation during sexual differentiation in fission yeast
- PMID: 18059475
- PMCID: PMC2206131
- DOI: 10.1038/sj.emboj.7601949
Cdc2p controls the forkhead transcription factor Fkh2p by phosphorylation during sexual differentiation in fission yeast
Abstract
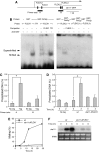
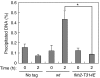
In most eukaryotes, cyclin-dependent kinases (Cdks) play a central role in control of cell-cycle progression. Cdks are inactivated from the end of mitosis to the start of the next cell cycle as well as during sexual differentiation. The forkhead-type transcription factor Fkh2p is required for the periodic expression of many genes and for efficient mating in the fission yeast Schizosaccharomyces pombe. However, the mechanism responsible for coordination of cell-cycle progression with sexual differentiation is still unknown. We now show that Fkh2p is phosphorylated by Cdc2p (Cdk1) and that phosphorylation of Fkh2p on T314 or S462 by this Cdk blocks mating in S. pombe by preventing the induction of ste11+ transcription, which is required for the onset of sexual development. We propose that functional interaction between Cdks and forkhead transcription factors may link the mitotic cell cycle and sexual differentiation.
Figures




Similar articles
-
Cdk phosphorylation of the Ste11 transcription factor constrains differentiation-specific transcription to G1.Genes Dev. 2007 Feb 1;21(3):347-59. doi: 10.1101/gad.407107. Genes Dev. 2007. PMID: 17289922 Free PMC article.
-
Fkh2p and Sep1p regulate mitotic gene transcription in fission yeast.J Cell Sci. 2004 Nov 1;117(Pt 23):5623-32. doi: 10.1242/jcs.01473. J Cell Sci. 2004. PMID: 15509866
-
Chemical genetic analysis of the regulatory role of Cdc2p in the S. pombe septation initiation network.J Cell Sci. 2008 Mar 15;121(Pt 6):843-53. doi: 10.1242/jcs.021584. Epub 2008 Feb 26. J Cell Sci. 2008. PMID: 18303049
-
Regulation of sexual differentiation initiation in Schizosaccharomyces pombe.Biosci Biotechnol Biochem. 2024 Apr 22;88(5):475-492. doi: 10.1093/bbb/zbae019. Biosci Biotechnol Biochem. 2024. PMID: 38449372 Review.
-
The central role of a CDK in controlling the fission yeast cell cycle.Harvey Lect. 1996-1997;92:55-64. Harvey Lect. 1996. PMID: 15372743 Review. No abstract available.
Cited by
-
TORC1 and TORC2 converge to regulate the SAGA co-activator in response to nutrient availability.EMBO Rep. 2017 Dec;18(12):2197-2218. doi: 10.15252/embr.201744942. Epub 2017 Oct 27. EMBO Rep. 2017. PMID: 29079657 Free PMC article.
-
Coupling TOR to the Cell Cycle by the Greatwall-Endosulfine-PP2A-B55 Pathway.Biomolecules. 2017 Aug 4;7(3):59. doi: 10.3390/biom7030059. Biomolecules. 2017. PMID: 28777780 Free PMC article. Review.
-
Novel TORC1 inhibitor Ecl1 is regulated by phosphorylation in fission yeast.Aging Cell. 2025 Apr;24(4):e14450. doi: 10.1111/acel.14450. Epub 2025 Feb 5. Aging Cell. 2025. PMID: 39910760 Free PMC article.
-
The putative forkhead transcription factor FhpA is necessary for development, aflatoxin production, and stress response in Aspergillus flavus.PLoS One. 2025 Mar 3;20(3):e0315766. doi: 10.1371/journal.pone.0315766. eCollection 2025. PLoS One. 2025. PMID: 40029854 Free PMC article.
-
Adding phosphorylation events to the core oscillator driving the cell cycle of fission yeast.PLoS One. 2018 Dec 4;13(12):e0208515. doi: 10.1371/journal.pone.0208515. eCollection 2018. PLoS One. 2018. PMID: 30513113 Free PMC article.
References
-
- Bahler J (2005) Cell-cycle control of gene expression in budding and fission yeast. Annu Rev Genet 39: 69–94 - PubMed
-
- Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A III, Steever AB, Wach A, Philippsen P, Pringle JR (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951 - PubMed
-
- Buck V, Ng SS, Ruiz-Garcia AB, Papadopoulou K, Bhatti S, Samuel JM, Anderson M, Millar JB, McInerny CJ (2004) Fkh2p and Sep1p regulate mitotic gene transcription in fission yeast. J Cell Sci 117: 5623–5632 - PubMed
-
- Costa RH (2005) FoxM1 dances with mitosis. Nat Cell Biol 7: 108–110 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

