Dimerization of FIR upon FUSE DNA binding suggests a mechanism of c-myc inhibition
- PMID: 18059478
- PMCID: PMC2206118
- DOI: 10.1038/sj.emboj.7601936
Dimerization of FIR upon FUSE DNA binding suggests a mechanism of c-myc inhibition
Abstract
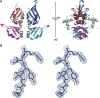
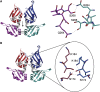
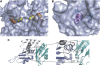
c-myc is essential for cell homeostasis and growth but lethal if improperly regulated. Transcription of this oncogene is governed by the counterbalancing forces of two proteins on TFIIH--the FUSE binding protein (FBP) and the FBP-interacting repressor (FIR). FBP and FIR recognize single-stranded DNA upstream of the P1 promoter, known as FUSE, and influence transcription by oppositely regulating TFIIH at the promoter site. Size exclusion chromatography coupled with light scattering reveals that an FIR dimer binds one molecule of single-stranded DNA. The crystal structure confirms that FIR binds FUSE as a dimer, and only the N-terminal RRM domain participates in nucleic acid recognition. Site-directed mutations of conserved residues in the first RRM domain reduce FIR's affinity for FUSE, while analogous mutations in the second RRM domain either destabilize the protein or have no effect on DNA binding. Oppositely oriented DNA on parallel binding sites of the FIR dimer results in spooling of a single strand of bound DNA, and suggests a mechanism for c-myc transcriptional control.
Figures





Similar articles
-
Quantitative characterization of the interactions among c-myc transcriptional regulators FUSE, FBP, and FIR.Biochemistry. 2010 Jun 8;49(22):4620-34. doi: 10.1021/bi9021445. Biochemistry. 2010. PMID: 20420426
-
The FUSE/FBP/FIR/TFIIH system is a molecular machine programming a pulse of c-myc expression.EMBO J. 2006 May 17;25(10):2119-30. doi: 10.1038/sj.emboj.7601101. Epub 2006 Apr 20. EMBO J. 2006. PMID: 16628215 Free PMC article.
-
The FBP interacting repressor targets TFIIH to inhibit activated transcription.Mol Cell. 2000 Feb;5(2):331-41. doi: 10.1016/s1097-2765(00)80428-1. Mol Cell. 2000. PMID: 10882074
-
[Alternative Splicing Detection as Biomarker Candidates for Cancer Diagnosis and Treatment with Establishment of Clinical Biobank in Chiba University].Rinsho Byori. 2015 Mar;63(3):347-60. Rinsho Byori. 2015. PMID: 26524858 Review. Japanese.
-
Far upstream element binding protein 1: a commander of transcription, translation and beyond.Oncogene. 2013 Jun 13;32(24):2907-16. doi: 10.1038/onc.2012.350. Epub 2012 Aug 27. Oncogene. 2013. PMID: 22926519 Free PMC article. Review.
Cited by
-
Predicting the involvement of polyQ- and polyA in protein-protein interactions by their amino acid context.Heliyon. 2024 Sep 14;10(18):e37861. doi: 10.1016/j.heliyon.2024.e37861. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39323775 Free PMC article.
-
Characterization of Protein-Nucleic Acid Complexes by Size-Exclusion Chromatography Coupled with Light Scattering, Absorbance, and Refractive Index Detectors.Methods Mol Biol. 2021;2263:381-395. doi: 10.1007/978-1-0716-1197-5_18. Methods Mol Biol. 2021. PMID: 33877609
-
Single-Stranded DNA Binding Proteins and Their Identification Using Machine Learning-Based Approaches.Biomolecules. 2022 Aug 26;12(9):1187. doi: 10.3390/biom12091187. Biomolecules. 2022. PMID: 36139026 Free PMC article. Review.
-
Oligomerization of the polycystin-2 C-terminal tail and effects on its Ca2+-binding properties.J Biol Chem. 2015 Apr 17;290(16):10544-54. doi: 10.1074/jbc.M115.641803. Epub 2015 Feb 25. J Biol Chem. 2015. PMID: 25716316 Free PMC article.
-
circMMD reduction following tumor treating fields inhibits glioblastoma progression through FUBP1/FIR/DVL1 and miR-15b-5p/FZD6 signaling.J Exp Clin Cancer Res. 2023 Mar 17;42(1):64. doi: 10.1186/s13046-023-02642-z. J Exp Clin Cancer Res. 2023. PMID: 36932454 Free PMC article.
References
-
- Allain FH, Gilbert DE, Bouvet P, Feigon J (2000) Solution structure of the two N-terminal RNA-binding domains of nucleolin and NMR study of the interaction with its RNA target. J Mol Biol 303: 227–241 - PubMed
-
- Amrute SB, Abdul-Manan Z, Pandey V, Williams KR, Modak MJ (1994) Purification and nucleic acid binding properties of a fragment of type C1/C2 heterogeneous nuclear ribonucleoprotein from thymic nuclear extracts. Biochemistry 33: 8282–8291 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

