Nonviral delivery of synthetic siRNAs in vivo
- PMID: 18060020
- PMCID: PMC2096447
- DOI: 10.1172/JCI33494
Nonviral delivery of synthetic siRNAs in vivo
Abstract
Sequence-specific gene silencing using small interfering RNA (siRNA) is a Nobel prize-winning technology that is now being evaluated in clinical trials as a potentially novel therapeutic strategy. This article provides an overview of the major pharmaceutical challenges facing siRNA therapeutics, focusing on the delivery strategies for synthetic siRNA duplexes in vivo, as this remains one of the most important issues to be resolved. This article also highlights the importance of understanding the genocompatibility/toxicogenomics of siRNA delivery reagents in terms of their impact on gene-silencing activity and specificity. Collectively, this information is essential for the selection of optimally acting siRNA delivery system combinations for the many proposed applications of RNA interference.
Figures

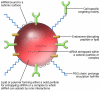
References
-
- Fire A., et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
-
- Kuhn R., Streif S., Wurst W. RNA interference in mice. Handb. Exp. Pharmacol. 2007;178:149–176. - PubMed
-
- Kim D.H., Rossi J.J. Strategies for silencing human disease using RNA interference. Nat. Rev. Genet. 2007;8:173–184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

