Therapeutic application of RNAi: is mRNA targeting finally ready for prime time?
- PMID: 18060021
- PMCID: PMC2096424
- DOI: 10.1172/JCI34129
Therapeutic application of RNAi: is mRNA targeting finally ready for prime time?
Abstract
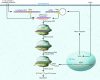
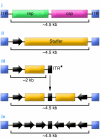
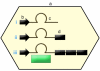
With unprecedented speed, RNA interference (RNAi) has advanced from its basic discovery in lower organisms to becoming a powerful genetic tool and perhaps our single most promising biotherapeutic for a wide array of diseases. Numerous studies document RNAi efficacy in laboratory animals, and the first clinical trials are underway and thus far suggest that RNAi is safe to use in humans. Yet substantial hurdles have also surfaced and must be surmounted before therapeutic RNAi applications can become a standard therapy. Here we review the most critical roadblocks and concerns for clinical RNAi transition, delivery, and safety. We highlight emerging solutions and concurrently discuss novel therapeutic RNAi-based concepts. The current rapid advances create realistic optimism that the establishment of RNAi as a new and potent clinical modality in humans is near.
Figures
References
-
- Elbashir S.M., et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. - PubMed
-
- McCaffrey A.P., et al. RNA interference in adult mice. Nature. 2002;418:38–39. - PubMed
-
- Dykxhoorn D.M., Lieberman J. The silent revolution: RNA interference as basic biology, research tool, and therapeutic. Annu. Rev. Med. 2005;56:401–423. - PubMed
-
- Hannon G.J., Rossi J.J. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371–378. - PubMed
-
- Kim D.H., Rossi J.J. Strategies for silencing human disease using RNA interference. Nat. Rev. Genet. 2007;8:173–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

