Microvascular destruction identifies murine allografts that cannot be rescued from airway fibrosis
- PMID: 18060031
- PMCID: PMC2096438
- DOI: 10.1172/JCI32311
Microvascular destruction identifies murine allografts that cannot be rescued from airway fibrosis
Abstract
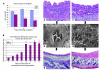
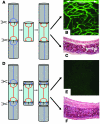
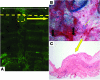
Small airway fibrosis (bronchiolitis obliterans syndrome) is the primary obstacle to long-term survival following lung transplantation. Here, we show the importance of functional microvasculature in the prevention of epithelial loss and fibrosis due to rejection and for the first time, relate allograft microvascular injury and loss of tissue perfusion to immunotherapy-resistant rejection. To explore the role of alloimmune rejection and airway ischemia in the development of fibroproliferation, we used a murine orthotopic tracheal transplant model. We determined that transplants were reperfused by connection of recipient vessels to donor vessels at the surgical anastomosis site. Microcirculation through the newly formed vascular anastomoses appeared partially dependent on VEGFR2 and CXCR2 pathways. In the absence of immunosuppression, the microvasculature in rejecting allografts exhibited vascular complement deposition, diminished endothelial CD31 expression, and absent perfusion prior to the onset of fibroproliferation. Rejecting grafts with extensive endothelial cell injury were refractory to immunotherapy. After early microvascular loss, neovascularization was eventually observed in the membranous trachea, indicating a reestablishment of graft perfusion in established fibrosis. One implication of this study is that bronchial artery revascularization at the time of lung transplantation may decrease the risk of subsequent airway fibrosis.
Figures


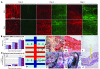
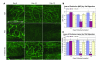
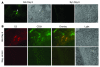
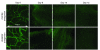
Comment in
-
Every allograft needs a silver lining.J Clin Invest. 2007 Dec;117(12):3645-8. doi: 10.1172/JCI34238. J Clin Invest. 2007. PMID: 18060023 Free PMC article.
References
-
- Trulock E.P., et al. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult lung and heart-lung transplantation report — 2006. J. Heart Lung Transplant. 2006;25:880–892. - PubMed
-
- Yousem S.A., Burke C.M., Billingham M.E. Pathologic pulmonary alterations in long-term human heart-lung transplantation. Hum. Pathol. 1985;16:911–923. - PubMed
-
- Sharples L.D., McNeil K., Stewart S., Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. . J. Heart Lung Transplant. 2002;21:271–281. - PubMed
-
- Luckraz H., et al. Microvascular changes in small airways predispose to obliterative bronchiolitis after lung transplantation. J. Heart Lung Transplant. 2004;23:527–531. - PubMed
-
- Luckraz H., et al. Is obliterative bronchiolitis in lung transplantation associated with microvascular damage to small airways? Ann. Thorac. Surg. 2006;82:1212–1218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

