Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons
- PMID: 18060037
- PMCID: PMC2104495
- DOI: 10.1172/JCI32829
Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons
Abstract
Sleep is a natural process that preserves energy, facilitates development, and restores the nervous system in higher animals. Sleep loss resulting from physiological and pathological conditions exerts tremendous pressure on neuronal circuitry responsible for sleep-wake regulation. It is not yet clear how acute and chronic sleep loss modify neuronal activities and lead to adaptive changes in animals. Here, we show that acute and chronic prolonged wakefulness in mice induced by modafinil treatment produced long-term potentiation (LTP) of glutamatergic synapses on hypocretin/orexin neurons in the lateral hypothalamus, a well-established arousal/wake-promoting center. A similar potentiation of synaptic strength at glutamatergic synapses on hypocretin/orexin neurons was also seen when mice were sleep deprived for 4 hours by gentle handling. Blockade of dopamine D1 receptors attenuated prolonged wakefulness and synaptic plasticity in these neurons, suggesting that modafinil functions through activation of the dopamine system. Also, activation of the cAMP pathway was not able to further induce LTP at glutamatergic synapses in brain slices from mice treated with modafinil. These results indicate that synaptic plasticity due to prolonged wakefulness occurs in circuits responsible for arousal and may contribute to changes in the brain and body of animals experiencing sleep loss.
Figures

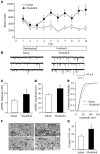

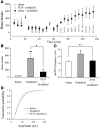

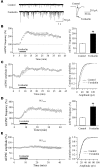

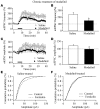
Comment in
-
Staying awake puts pressure on brain arousal systems.J Clin Invest. 2007 Dec;117(12):3648-50. doi: 10.1172/JCI34250. J Clin Invest. 2007. PMID: 18060024 Free PMC article.
References
-
- Tononi G., Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10:49–62. - PubMed
-
- Rechtschaffen A., Bergmann B.M., Everson C.A., Kushida C.A., Gilliland M.A. Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep. 1989;12:68–87. - PubMed
-
- Bonnet M.H., Arand D.L. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med. Rev. 2003;7:297–310. - PubMed
-
- Durmer J.S., Dinges D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2005;25:117–129. - PubMed

