Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice
- PMID: 18060047
- PMCID: PMC2104477
- DOI: 10.1172/JCI32273
Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice
Abstract
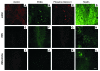
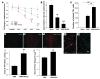
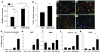
Dopamine (DA) cell replacement therapy in Parkinson disease (PD) can be achieved using human fetal mesencephalic tissue; however, limited tissue availability has hindered further developments. Embryonic stem cells provide a promising alternative, but poor survival and risk of teratoma formation have prevented their clinical application. We present here a method for generating large numbers of DA neurons based on expanding and differentiating ventral midbrain (VM) neural stem cells/progenitors in the presence of key signals necessary for VM DA neuron development. Mouse VM neurospheres (VMNs) expanded with FGF2, differentiated with sonic hedgehog and FGF8, and transfected with Wnt5a (VMN-Wnt5a) generated 10-fold more DA neurons than did conventional FGF2-treated VMNs. VMN-Wnt5a cells exhibited the transcriptional and biochemical profiles and intrinsic electrophysiological properties of midbrain DA cells. Transplantation of these cells into parkinsonian mice resulted in significant cellular and functional recovery. Importantly, no tumors were detected and only a few transplanted grafts contained sporadic nestin-expressing progenitors. Our findings show that Wnt5a improves the differentiation and functional integration of stem cell-derived DA neurons in vivo and define Wnt5a-treated neural stem cells as an efficient and safe source of DA neurons for cell replacement therapy in PD.
Figures




Similar articles
-
Wnt5a regulates ventral midbrain morphogenesis and the development of A9-A10 dopaminergic cells in vivo.PLoS One. 2008;3(10):e3517. doi: 10.1371/journal.pone.0003517. Epub 2008 Oct 27. PLoS One. 2008. PMID: 18953410 Free PMC article.
-
Efficient expansion and dopaminergic differentiation of human fetal ventral midbrain neural stem cells by midbrain morphogens.Neurobiol Dis. 2013 Jan;49:118-27. doi: 10.1016/j.nbd.2012.08.006. Epub 2012 Aug 24. Neurobiol Dis. 2013. PMID: 22940632
-
Isolation of LMX1a Ventral Midbrain Progenitors Improves the Safety and Predictability of Human Pluripotent Stem Cell-Derived Neural Transplants in Parkinsonian Disease.J Neurosci. 2019 Nov 27;39(48):9521-9531. doi: 10.1523/JNEUROSCI.1160-19.2019. Epub 2019 Oct 22. J Neurosci. 2019. PMID: 31641054 Free PMC article.
-
Engineering a dopaminergic phenotype in stem/precursor cells: role of Nurr1, glia-derived signals, and Wnts.Ann N Y Acad Sci. 2005 May;1049:51-66. doi: 10.1196/annals.1334.007. Ann N Y Acad Sci. 2005. PMID: 15965107 Review.
-
Stem cell potential in Parkinson's disease and molecular factors for the generation of dopamine neurons.Biochim Biophys Acta. 2011 Jan;1812(1):1-11. doi: 10.1016/j.bbadis.2010.08.006. Epub 2010 Aug 14. Biochim Biophys Acta. 2011. PMID: 20713152 Review.
Cited by
-
Direct in vivo assessment of human stem cell graft-host neural circuits.Neuroimage. 2015 Jul 1;114:328-37. doi: 10.1016/j.neuroimage.2015.03.079. Epub 2015 Apr 25. Neuroimage. 2015. PMID: 25936696 Free PMC article.
-
Wnt2 regulates progenitor proliferation in the developing ventral midbrain.J Biol Chem. 2010 Mar 5;285(10):7246-53. doi: 10.1074/jbc.M109.079822. Epub 2009 Dec 16. J Biol Chem. 2010. PMID: 20018874 Free PMC article.
-
Interactions of Wnt/beta-catenin signaling and sonic hedgehog regulate the neurogenesis of ventral midbrain dopamine neurons.J Neurosci. 2010 Jul 7;30(27):9280-91. doi: 10.1523/JNEUROSCI.0860-10.2010. J Neurosci. 2010. PMID: 20610763 Free PMC article.
-
LncRNA H19 diminishes dopaminergic neuron loss by mediating microRNA-301b-3p in Parkinson's disease via the HPRT1-mediated Wnt/β-catenin signaling pathway.Aging (Albany NY). 2020 May 20;12(10):8820-8836. doi: 10.18632/aging.102877. Epub 2020 May 20. Aging (Albany NY). 2020. PMID: 32434961 Free PMC article.
-
The potential role of neuroinflammation and transcription factors in Parkinson disease.Dialogues Clin Neurosci. 2017 Mar;19(1):71-80. doi: 10.31887/DCNS.2017.19.1/rpal. Dialogues Clin Neurosci. 2017. PMID: 28566949 Free PMC article. Review.
References
-
- Kordower J.H., et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N. Engl. J. Med. 1995;332:1118–1124. - PubMed
-
- Piccini P., et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat. Neurosci. 1999;2:1137–1140. - PubMed
-
- Piccini P., et al. Delayed recovery of movement-related cortical function in Parkinson’s disease after striatal dopaminergic grafts. Ann. Neurol. 2000;48:689–695. - PubMed
-
- Olanow C.W., et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003;54:403–414. - PubMed
-
- Freed C.R., et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 2001;344:710–719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

