Effects of blood collection conditions on ovarian cancer serum markers
- PMID: 18060075
- PMCID: PMC2093996
- DOI: 10.1371/journal.pone.0001281
Effects of blood collection conditions on ovarian cancer serum markers
Abstract
Background: Evaluating diagnostic and early detection biomarkers requires comparing serum protein concentrations among biosamples ascertained from subjects with and without cancer. Efforts are generally made to standardize blood processing and storage conditions for cases and controls, but blood sample collection conditions cannot be completely controlled. For example, blood samples from cases are often obtained from persons aware of their diagnoses, and collected after fasting or in surgery, whereas blood samples from some controls may be obtained in different conditions, such as a clinic visit. By measuring the effects of differences in collection conditions on three different markers, we investigated the potential of these effects to bias validation studies.
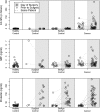
Methodology and principle findings: We analyzed serum concentrations of three previously studied putative ovarian cancer serum biomarkers-CA 125, Prolactin and MIF-in healthy women, women with ovarian cancer undergoing gynecologic surgery, women undergoing surgery for benign ovary pathology, and women undergoing surgery with pathologically normal ovaries. For women undergoing surgery, a blood sample was collected either in the clinic 1 to 39 days prior to surgery, or on the day of surgery after anesthesia was administered but prior to the surgical procedure, or both. We found that one marker, prolactin, was dramatically affected by collection conditions, while CA 125 and MIF were unaffected. Prolactin levels were not different between case and control groups after accounting for the conditions of sample collection, suggesting that sample ascertainment could explain some or all of the previously reported results about its potential as a biomarker for ovarian cancer.
Conclusions: Biomarker validation studies should use standardized collection conditions, use multiple control groups, and/or collect samples from cases prior to influence of diagnosis whenever feasible to detect and correct for potential biases associated with sample collection.
Conflict of interest statement
Figures

References
-
- Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. - PubMed
-
- Pepe M. Evaluating technologies for classification and prediction in medicine. Statistics in Medicine. 2005;24:3687–3696. - PubMed
-
- Rosenthal AN, Menon U, Jacobs IJ. Screening for ovarian cancer. Clin Obstet Gynecol. 2006;49:433–447. - PubMed
-
- Pauler DK, Menon U, McIntosh M, Symecko HL, Skates SJ, et al. Factors Influencing Serum CA125II Levels in Healthy Postmenopausal Women. Cancer Epidemiol Biomarkers Prev. 2001;10:489–493. - PubMed
-
- Skates SJ, Menon U, MacDonald N, Rosenthal AN, Oram DH, et al. Calculation of the Risk of Ovarian Cancer From Serial CA-125 Values for Preclinical Detection in Postmenopausal Women. J Clin Oncol. 2003;21:206s–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

