Pre-receptor regulation of the androgen receptor
- PMID: 18060684
- PMCID: PMC2225387
- DOI: 10.1016/j.mce.2007.10.008
Pre-receptor regulation of the androgen receptor
Abstract
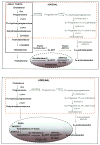
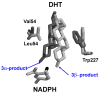
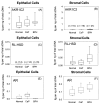
The human androgen receptor (AR) is a ligand-activated nuclear transcription factor and mediates the induction of genes involved in the development of the male phenotype and male secondary sex characteristics, as well as the normal and abnormal growth of the prostate. We have identified the pair of hydroxysteroid dehydrogenases (HSDs) that regulate ligand access to the AR in human prostate. We find that type 3 3alpha-HSD (aldo-keto reductase (AKR)1C2) catalyzes the NADPH dependent reduction of the potent androgen 5alpha-dihydrotestosterone (5alpha-DHT) to yield the inactive androgen 3alpha-androstanediol (3alpha-diol). We also find that RoDH like 3alpha-HSD (RL-HSD) catalyzes the NAD(+) dependent oxidation of 3alpha-diol to yield 5alpha-DHT. Together these enzymes are involved in the pre-receptor regulation of androgen action. Inhibition of AKR1C2 would be desirable in cases of androgen insufficiency and inhibition of RL-HSD might be desirable in benign prostatic hyperplasia.
Figures


References
-
- Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends in Endocrinology & Metabolism. 2004;15:432–438. - PubMed
-
- Bauman DR, Steckelbroeck S, Peehl DM, Penning TM. Transcript profiling of the androgen signal in normal prostate, benign prostatic hyperplasia, and prostate cancer. Endocrinology. 2006a;147:5806–5816. - PubMed
-
- Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3α-hydroxysteroid dehydrogenase in human prostate that converts 5α-androstane-3α,17β-diol to 5α-dihydrotestosterone: A potential therapeutic target for androgen dependent disease. Mol Endocrinol. 2006b;20:444–458. - PubMed
-
- Biswas MG, Russell DW. Expression cloning and characterization of oxidative 17β- and 3α-hydroxysteroid dehydrogenases from rat and human prostate. J Biol Chem. 1997;272:15959–15966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

