Nociceptive neurons protect Drosophila larvae from parasitoid wasps
- PMID: 18060782
- PMCID: PMC2225350
- DOI: 10.1016/j.cub.2007.11.029
Nociceptive neurons protect Drosophila larvae from parasitoid wasps
Erratum in
- Curr Biol. 2007 Dec 18;17(24):2183
Abstract
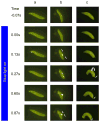
Background: Natural selection has resulted in a complex and fascinating repertoire of innate behaviors that are produced by insects. One puzzling example occurs in fruit fly larvae that have been subjected to a noxious mechanical or thermal sensory input. In response, the larvae "roll" with a motor pattern that is completely distinct from the style of locomotion that is used for foraging.
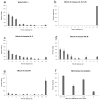
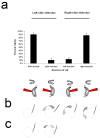
Results: We have precisely mapped the sensory neurons that are used by the Drosophila larvae to detect nociceptive stimuli. By using complementary optogenetic activation and targeted silencing of sensory neurons, we have demonstrated that a single class of neuron (class IV multidendritic neuron) is sufficient and necessary for triggering the unusual rolling behavior. In addition, we find that larvae have an innately encoded preference in the directionality of rolling. Surprisingly, the initial direction of rolling locomotion is toward the side of the body that has been stimulated. We propose that directional rolling might provide a selective advantage in escape from parasitoid wasps that are ubiquitously present in the natural environment of Drosophila. Consistent with this hypothesis, we have documented that larvae can escape the attack of Leptopilina boulardi parasitoid wasps by rolling, occasionally flipping the attacker onto its back.
Conclusions: The class IV multidendritic neurons of Drosophila larvae are nociceptive. The nociception behavior of Drosophila melanagaster larvae includes an innately encoded directional preference. Nociception behavior is elicited by the ecologically relevant sensory stimulus of parasitoid wasp attack.
Figures



Comment in
-
Neuroethology: a neuronal self-defense mechanism in fly larvae.Curr Biol. 2008 Feb 12;18(3):R116-7. doi: 10.1016/j.cub.2007.11.054. Curr Biol. 2008. PMID: 18269904
References
-
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. - PubMed
-
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. - PubMed
-
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. - PubMed
-
- Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–785. - PubMed
-
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
