Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling
- PMID: 18061204
- PMCID: PMC2761085
- DOI: 10.1016/j.yjmcc.2007.10.012
Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling
Abstract
The close relationship between life-threatening ventricular arrhythmias and contractile dysfunction in the heart implicates intracellular calcium cycling as an important underlying mechanism of arrhythmogenesis. Despite this close association, however, the mechanisms of arrhythmogenesis attributable to impaired calcium cycling are not fully appreciated or understood. In this report we review some of the current thinking regarding arrhythmia mechanisms associated with either abnormal impulse initiation (i.e. arrhythmia triggers) or impulse propagation (i.e. arrhythmia substrates). In all cases, the mechanisms are primarily related to dysfunction of calcium regulatory proteins associated with the sarcomere. These findings highlight the broad scope of arrhythmias associated with abnormal calcium cycling, and provide a basis for a causal relationship between cardiac electrical instability and contractile dysfunction. Moreover, calcium cycling proteins may provide much needed targets for novel antiarrhythmic therapies.
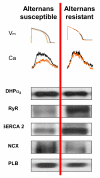
Figures
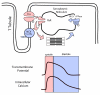

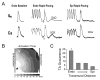
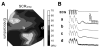
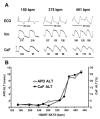

References
-
- Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. - PubMed
-
- Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–8. - PubMed
-
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. - PubMed
-
- Tomaselli GF, Marbán E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc.Res. 1999;42:270–283. - PubMed
-
- Pruvot E, Katra RP, Rosenbaum DS, Laurita KR. Calcium cycling as a mechanism of repolarization alternans onset in the intact heart. Circulation. 2002;19:11–191. Abstract.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

