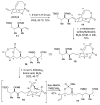
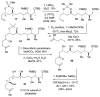
A multifaceted phosphate tether: application to the C1-C14 subunit of dolabelides A-D
- PMID: 18062695
- PMCID: PMC2640944
- DOI: 10.1021/ol7025944
A multifaceted phosphate tether: application to the C1-C14 subunit of dolabelides A-D
Abstract
A phosphate tether approach to the C1-14 subunit of dolabelide is described. The phosphate tether serves a multifaceted role mediating several processes, including (i) diastereotopic differentiation via RCM, (ii) selective CM by imparting Type III behavior to the exocyclic olefin, (iii) regioselective hydrogenation, and (iv) regioselective Pd(0)-catalyzed reductive opening of the bicyclic phosphate. Overall, this strategy uses orthogonal protecting- and leaving-group properties innate to phosphate esters to rapidly assembly the titled subunit.
Figures
Similar articles
-
Total synthesis of dolabelide C: a phosphate-mediated approach.J Org Chem. 2011 Jun 3;76(11):4358-70. doi: 10.1021/jo2003506. Epub 2011 Apr 29. J Org Chem. 2011. PMID: 21528846 Free PMC article.
-
A multifaceted phosphate tether: application to the C15-C30 subunit of dolabelides A-D.Org Lett. 2008 Apr 3;10(7):1421-4. doi: 10.1021/ol8001865. Epub 2008 Mar 7. Org Lett. 2008. PMID: 18324822 Free PMC article.
-
Total synthesis of dolabelide D.J Am Chem Soc. 2006 Mar 8;128(9):2796-7. doi: 10.1021/ja058692k. J Am Chem Soc. 2006. PMID: 16506747 Free PMC article.
-
Phosphate tethers in natural product synthesis.Top Curr Chem. 2015;361:253-71. doi: 10.1007/128_2014_572. Top Curr Chem. 2015. PMID: 25518970 Review.
-
BINOL-derived phosphoramidites in asymmetric hydrogenation: can the presence of a functionality in the amino group influence the catalytic outcome?Chem Soc Rev. 2008 Apr;37(4):839-64. doi: 10.1039/b714519e. Epub 2008 Feb 18. Chem Soc Rev. 2008. PMID: 18362987 Review.
Cited by
-
Total synthesis of dolabelide C: a phosphate-mediated approach.J Org Chem. 2011 Jun 3;76(11):4358-70. doi: 10.1021/jo2003506. Epub 2011 Apr 29. J Org Chem. 2011. PMID: 21528846 Free PMC article.
-
Crotylboron-based synthesis of the polypropionate units of chaxamycins A/D, salinisporamycin, and rifamycin S.J Org Chem. 2013 Jan 4;78(1):3-8. doi: 10.1021/jo3008226. Epub 2012 Jun 15. J Org Chem. 2013. PMID: 22703288 Free PMC article.
-
A concise, phosphate-mediated approach to the total synthesis of (-)-tetrahydrolipstatin.Org Lett. 2010 Apr 2;12(7):1556-9. doi: 10.1021/ol1002913. Org Lett. 2010. PMID: 20196547 Free PMC article.
-
A multifaceted phosphate tether: application to the C15-C30 subunit of dolabelides A-D.Org Lett. 2008 Apr 3;10(7):1421-4. doi: 10.1021/ol8001865. Epub 2008 Mar 7. Org Lett. 2008. PMID: 18324822 Free PMC article.
-
Phosphate tether-mediated approach to the formal total synthesis of (-)-salicylihalamides A and B.J Org Chem. 2011 May 20;76(10):3909-16. doi: 10.1021/jo200337v. Epub 2011 Apr 19. J Org Chem. 2011. PMID: 21504150 Free PMC article.
References
-
- Ojika M, Nagoya T, Yamada K. Tetrahedron Lett. 1995;36:7491–7494.
-
- Suenaga K, Nagoya T, Shibata T, Kigoshi H, Yamada K. J. Nat. Prod. 1997;60:155–157.
-
- Grimaud L, Rotulo D, Ros-Perez R, Guitry-Azam L, Prunet J. Tetrahedron Lett. 2002;43:7477–7479.
- Grimaud L, de Mesmay R, Prunet J. Org. Lett. 2002;4:419–421. - PubMed
- Desroy N, Le Roux R, Phansavath P, Chiummiento L, Bonini C, Genet J-P. Tetrahedron Lett. 2003;44:1763–1766.
- Schmidt DR, Park PK, Leighton JL. Org. Lett. 2003;5:3535–3537. - PubMed
- Le Roux R, Desroy N, Phansavath P, Genêt J-P. Synlett. 2005:429–432.
- Keck GE, McLaws MD. Tetrahedron Lett. 2005;46:4911–4914. - PMC - PubMed
- Vincent A, Prunet J. Synlett. 2006:2269–2271.
-
- Rubinstenn G, Esnault J, Mallet J-M, Sinay P. Tetrahedron: Asymmetry. 1997;8:1327–1336. For examples of P(III)/P(V)-based tethers in synthesis, see.
- Sprott KT, McReynolds MD, Hanson PR. Org. Lett. 2001;3:3939–3942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources