Characterization of a human coagulation factor Xa-binding site on Viperidae snake venom phospholipases A2 by affinity binding studies and molecular bioinformatics
- PMID: 18062812
- PMCID: PMC2248580
- DOI: 10.1186/1472-6807-7-82
Characterization of a human coagulation factor Xa-binding site on Viperidae snake venom phospholipases A2 by affinity binding studies and molecular bioinformatics
Abstract
Background: The snake venom group IIA secreted phospholipases A2 (SVPLA2), present in the Viperidae snake family exhibit a wide range of toxic and pharmacological effects. They exert their different functions by catalyzing the hydrolysis of phospholipids (PL) at the membrane/water interface and by highly specific direct binding to: (i) presynaptic membrane-bound or intracellular receptors; (ii) natural PLA2-inhibitors from snake serum; and (iii) coagulation factors present in human blood.
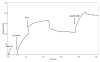
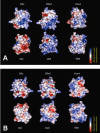
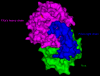
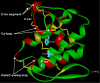
Results: Using surface plasmon resonance (SPR) protein-protein interaction measurements and an in vitro biological test of inhibition of prothrombinase activity, we identify a number of Viperidae venom SVPLA2s that inhibit blood coagulation through direct binding to human blood coagulation factor Xa (FXa) via a non-catalytic, PL-independent mechanism. We classify the SVPLA2s in four groups, depending on the strength of their binding. Molecular electrostatic potentials calculated at the surface of 3D homology-modeling models show a correlation with inhibition of prothrombinase activity. In addition, molecular docking simulations between SVPLA2 and FXa guided by the experimental data identify the potential FXa binding site on the SVPLA2s. This site is composed of the following regions: helices A and B, the Ca2+ loop, the helix C-beta-wing loop, and the C-terminal fragment. Some of the SVPLA2 binding site residues belong also to the interfacial binding site (IBS). The interface in FXa involves both, the light and heavy chains.
Conclusion: We have experimentally identified several strong FXa-binding SVPLA2s that disrupt the function of the coagulation cascade by interacting with FXa by the non-catalytic PL-independent mechanism. By theoretical methods we mapped the interaction sites on both, the SVPLA2s and FXa. Our findings may lead to the design of novel, non-competitive FXa inhibitors.
Figures


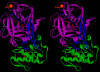




References
-
- Davie EW. Biochemical and molecular aspects of the coagulation cascade. Thromb Haemost. 1995;74:1–6. - PubMed
-
- Sabharwal AK, Padmanabhan K, Tulinsky A, Mathur A, Gorka J, Bajaj SP. Interaction of calcium with native and decarboxylated human factor X. Effect of proteolysis in the autolysis loop on catalytic efficiency and factor Va binding. J Biol Chem. 1997;272:22037–22045. doi: 10.1074/jbc.272.35.22037. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

