Nuclear RNA export factor 7 is localized in processing bodies and neuronal RNA granules through interactions with shuttling hnRNPs
- PMID: 18063567
- PMCID: PMC2241847
- DOI: 10.1093/nar/gkm556
Nuclear RNA export factor 7 is localized in processing bodies and neuronal RNA granules through interactions with shuttling hnRNPs
Abstract
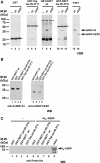
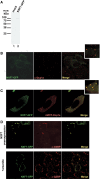
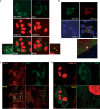
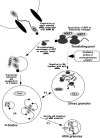
The nuclear RNA export factor (NXF) family proteins have been implicated in various aspects of post-transcriptional gene expression. This study shows that mouse NXF7 exhibits heterologous localization, i.e. NXF7 associates with translating ribosomes, stress granules (SGs) and processing bodies (P-bodies), the latter two of which are believed to be cytoplasmic sites of storage, degradation and/or sorting of mRNAs. By yeast two-hybrid screening, a series of heterogeneous nuclear ribonucleoproteins (hnRNPs) were identified as possible binding partners for NXF7. Among them, hnRNP A3, which is believed to be involved in translational control and/or cytoplasmic localization of certain mRNAs, formed a stable complex with NXF7 in vitro. Although hnRNP A3 was not associated with translating ribosomes, it was co-localized with NXF7 in P-bodies. After exposing to oxidative stress, NXF7 trans-localized to SGs, whereas hnRNP A3 did not. In differentiated neuroblastoma Neuro2a cells, NXF7 was co-localized with hnRNP A3 in cell body and neurites. The amino terminal half of NXF7, which was required for stable complex formation with hnRNP A3, coincided with the region required for localization in both P-bodies and neuronal RNA granules. These findings suggest that NXF7 plays a role in sorting, transport and/or storage of mRNAs through interactions with hnRNP A3.
Figures





References
-
- Gruter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber BK, Izaurralde E. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell. 1998;1:649–659. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

