Effect of European Clinical Trials Directive on academic drug trials in Denmark: retrospective study of applications to the Danish Medicines Agency 1993-2006
- PMID: 18063611
- PMCID: PMC2174768
- DOI: 10.1136/bmj.39401.470648.BE
Effect of European Clinical Trials Directive on academic drug trials in Denmark: retrospective study of applications to the Danish Medicines Agency 1993-2006
Abstract
Objective: To determine the impact of the European Union's Clinical Trials Directive on the number of academic drug trials carried out in Denmark.
Design: Retrospective review of applications for drug trials to the Danish Medicines Agency, 1993-2006.
Review methods: Applications for drug trials for alternate years were classified as academic or commercial trials. A random subset of academic trials was reviewed for number of participants in and intended monitoring of the trials.
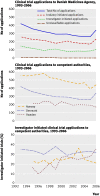
Results: Academic and commercial drug trials showed an identical steady decline from 1993 to 2006 and no noticeable change after 2004 when good clinical practice became mandatory for academic trials.
Conclusion: The Clinical Trials Directive introduced in May 2004 to ensure good clinical practice for academic drug trials was not associated with a decline in research activity in Denmark; presumably because good clinical practice units had already been in place in Danish universities since 1999. With such an infrastructure academic researchers can do drug trials under the same regulations as drug companies.
Conflict of interest statement
Competing interests: None declared.
Figures
Republished in
-
[Effect of the GCP Directive on academic drug trials].Ugeskr Laeger. 2008 Aug 11;170(33):2437-9. Ugeskr Laeger. 2008. PMID: 18761825 Danish.
References
-
- Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Official J Eur Commun 2001;L121:34-44. - PubMed
-
- Morice AH. The death of academic clinical trials. Lancet 2003;361:1568 - PubMed
-
- European Commission. European clinical trials database. http://eudract.emea.europa.eu
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
