Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurones
- PMID: 18063661
- PMCID: PMC2375643
- DOI: 10.1113/jphysiol.2007.145375
Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurones
Abstract
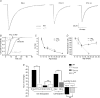
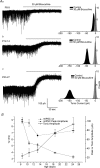
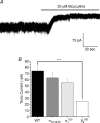
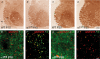
Thalamic ventrobasal (VB) relay neurones express multiple GABA(A) receptor subtypes mediating phasic and tonic inhibition. During postnatal development, marked changes in subunit expression occur, presumably reflecting changes in functional properties of neuronal networks. The aims of this study were to characterize the properties of synaptic and extrasynaptic GABA(A) receptors of developing VB neurones and investigate the role of the alpha(1) subunit during maturation of GABA-ergic transmission, using electrophysiology and immunohistochemistry in wild type (WT) and alpha(1)(0/0) mice and mice engineered to express diazepam-insensitive receptors (alpha(1H101R), alpha(2H101R)). In immature brain, rapid (P8/9-P10/11) developmental change to mIPSC kinetics and increased expression of extrasynaptic receptors (P8-27) formed by the alpha(4) and delta subunit occurred independently of the alpha(1) subunit. Subsequently (> or = P15), synaptic alpha(2) subunit/gephyrin clusters of WT VB neurones were replaced by those containing the alpha(1) subunit. Surprisingly, in alpha(1)(0/0) VB neurones the frequency of mIPSCs decreased between P12 and P27, because the alpha(2) subunit also disappeared from these cells. The loss of synaptic GABA(A) receptors led to a delayed disruption of gephyrin clusters. Despite these alterations, GABA-ergic terminals were preserved, perhaps maintaining tonic inhibition. These results demonstrate that maturation of synaptic and extrasynaptic GABA(A) receptors in VB follows a developmental programme independent of the alpha(1) subunit. Changes to synaptic GABA(A) receptor function and the increased expression of extrasynaptic GABA(A) receptors represent two distinct mechanisms for fine-tuning GABA-ergic control of thalamic relay neurone activity during development.
Figures







Similar articles
-
Developmentally regulated neurosteroid synthesis enhances GABAergic neurotransmission in mouse thalamocortical neurones.J Physiol. 2015 Jan 1;593(1):267-84. doi: 10.1113/jphysiol.2014.280263. Epub 2014 Dec 3. J Physiol. 2015. PMID: 25556800 Free PMC article.
-
The expression of GABAA beta subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells.J Physiol. 2008 Feb 15;586(4):989-1004. doi: 10.1113/jphysiol.2007.146746. Epub 2007 Dec 13. J Physiol. 2008. PMID: 18079158 Free PMC article.
-
An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons.J Neurophysiol. 2005 Dec;94(6):4491-501. doi: 10.1152/jn.00421.2005. Epub 2005 Sep 14. J Neurophysiol. 2005. PMID: 16162835
-
GABAA receptors in the thalamus: alpha4 subunit expression and alcohol sensitivity.Alcohol. 2007 May;41(3):177-85. doi: 10.1016/j.alcohol.2007.03.010. Epub 2007 May 23. Alcohol. 2007. PMID: 17521848 Review.
-
Endogenous neurosteroids influence synaptic GABAA receptors during postnatal development.J Neuroendocrinol. 2018 Feb;30(2). doi: 10.1111/jne.12537. J Neuroendocrinol. 2018. PMID: 28905487 Review.
Cited by
-
Realising the therapeutic potential of neuroactive steroid modulators of the GABAA receptor.Neurobiol Stress. 2019 Dec 23;12:100207. doi: 10.1016/j.ynstr.2019.100207. eCollection 2020 May. Neurobiol Stress. 2019. PMID: 32435660 Free PMC article.
-
Optogenetic activation of nigral inhibitory inputs to motor thalamus in the mouse reveals classic inhibition with little potential for rebound activation.Front Cell Neurosci. 2014 Feb 11;8:36. doi: 10.3389/fncel.2014.00036. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24574972 Free PMC article.
-
During postnatal development endogenous neurosteroids influence GABA-ergic neurotransmission of mouse cortical neurons.Neuropharmacology. 2016 Apr;103:163-73. doi: 10.1016/j.neuropharm.2015.11.019. Epub 2015 Dec 2. Neuropharmacology. 2016. PMID: 26626485 Free PMC article.
-
Postnatal development of synaptic properties of the GABAergic projection from the inferior colliculus to the auditory thalamus.J Neurophysiol. 2013 Jun;109(12):2866-82. doi: 10.1152/jn.00021.2013. Epub 2013 Mar 27. J Neurophysiol. 2013. PMID: 23536710 Free PMC article.
-
Developmentally regulated neurosteroid synthesis enhances GABAergic neurotransmission in mouse thalamocortical neurones.J Physiol. 2015 Jan 1;593(1):267-84. doi: 10.1113/jphysiol.2014.280263. Epub 2014 Dec 3. J Physiol. 2015. PMID: 25556800 Free PMC article.
References
-
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. - PubMed
-
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience. 2006;138:821–829. - PubMed
-
- Benson JA, Low K, Keist R, Mohler H, Rudolph U. Pharmacology of recombinant γ-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated α-subunits. FEBS Lett. 1998;431:400–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

