Accumulation of cytoplasmic calcium, but not apamin-sensitive afterhyperpolarization current, during high frequency firing in rat subthalamic nucleus cells
- PMID: 18063664
- PMCID: PMC2375605
- DOI: 10.1113/jphysiol.2007.141929
Accumulation of cytoplasmic calcium, but not apamin-sensitive afterhyperpolarization current, during high frequency firing in rat subthalamic nucleus cells
Abstract
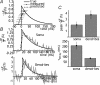
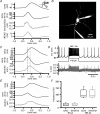
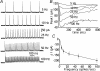
The autonomous firing pattern of neurons in the rat subthalamic nucleus (STN) is shaped by action potential afterhyperpolarization currents. One of these is an apamin-sensitive calcium-dependent potassium current (SK). The duration of SK current is usually considered to be limited by the clearance of calcium from the vicinity of the channel. When the cell is driven to fire faster, calcium is expected to accumulate, and this is expected to result in accumulation of calcium-dependent AHP current. We measured the time course of calcium transients in the soma and proximal dendrites of STN neurons during spontaneous firing and their accumulation during driven firing. We compared these to the time course and accumulation of AHP currents using whole-cell and perforated patch recordings. During spontaneous firing, a rise in free cytoplasmic calcium was seen after each action potential, and decayed with a time constant of about 200 ms in the soma, and 80 ms in the dendrites. At rates higher than 10 Hz, calcium transients accumulated as predicted. In addition, there was a slow calcium transient not predicted by summation of action potentials that became more pronounced at high firing frequency. Spike AHP currents were measured in voltage clamp as tail currents after 2 ms voltage pulses that triggered action currents. Apamin-sensitive AHP (SK) current was measured by subtraction of tail currents obtained before and after treatment with apamin. SK current peaked between 10 and 15 ms after an action potential, had a decay time constant of about 30 ms, and showed no accumulation. At frequencies between 5 and 200 spikes s(-1), the maximal SK current remained the same as that evoked by a single action potential. AHP current did not have time to decay between action potentials, so at frequencies above 50 spikes s(-1) the apamin-sensitive current was effectively constant. These results are inconsistent with the view that the decay of SK current is governed by calcium dynamics. They suggest that the calcium is present at the SK channel for a very short time after each action potential, and the current decays at a rate set by the deactivation kinetics of the SK channel. At high rates, repetitive firing was governed by a fast apamin-insensitive AHP current that did not accumulate, but rather showed depression with increases in activation frequency. A slowly accumulating AHP current, also insensitive to apamin, was extremely small at low rates but became significant with higher firing rates.
Figures







Similar articles
-
Apamin-sensitive small conductance calcium-activated potassium channels, through their selective coupling to voltage-gated calcium channels, are critical determinants of the precision, pace, and pattern of action potential generation in rat subthalamic nucleus neurons in vitro.J Neurosci. 2003 Aug 20;23(20):7525-42. doi: 10.1523/JNEUROSCI.23-20-07525.2003. J Neurosci. 2003. PMID: 12930791 Free PMC article.
-
Small conductance Ca2+-activated K+ channels regulate firing properties and excitability in parasympathetic cardiac motoneurons in the nucleus ambiguus.Am J Physiol Cell Physiol. 2010 Dec;299(6):C1285-98. doi: 10.1152/ajpcell.00134.2010. Epub 2010 Aug 25. Am J Physiol Cell Physiol. 2010. PMID: 20739619 Free PMC article.
-
Physiological role of calcium-activated potassium currents in the rat lateral amygdala.J Neurosci. 2002 Mar 1;22(5):1618-28. doi: 10.1523/JNEUROSCI.22-05-01618.2002. J Neurosci. 2002. PMID: 11880492 Free PMC article.
-
Small-conductance calcium-activated potassium channels.Ann N Y Acad Sci. 1999 Apr 30;868:370-8. doi: 10.1111/j.1749-6632.1999.tb11298.x. Ann N Y Acad Sci. 1999. PMID: 10414306 Review.
-
Molecular and cellular basis of small--and intermediate-conductance, calcium-activated potassium channel function in the brain.Cell Mol Life Sci. 2008 Oct;65(20):3196-217. doi: 10.1007/s00018-008-8216-x. Cell Mol Life Sci. 2008. PMID: 18597044 Free PMC article. Review.
Cited by
-
Predicting the responses of repetitively firing neurons to current noise.PLoS Comput Biol. 2014 May 8;10(5):e1003612. doi: 10.1371/journal.pcbi.1003612. eCollection 2014 May. PLoS Comput Biol. 2014. PMID: 24809636 Free PMC article.
-
Oscillators and Oscillations in the Basal Ganglia.Neuroscientist. 2015 Oct 1;21(5):530-539. doi: 10.1177/1073858414560826. Epub 2014 Dec 1. Neuroscientist. 2015. PMID: 25449134 Free PMC article. Review.
-
Optimization of an anatomically and electrically detailed rodent subthalamic nucleus neuron model.J Neurophysiol. 2024 Jul 1;132(1):136-146. doi: 10.1152/jn.00287.2023. Epub 2024 Jun 12. J Neurophysiol. 2024. PMID: 38863430 Free PMC article.
-
The role of SK calcium-dependent potassium currents in regulating the activity of deep cerebellar nucleus neurons: a dynamic clamp study.Cerebellum. 2008;7(4):542-6. doi: 10.1007/s12311-008-0077-1. Cerebellum. 2008. PMID: 18985424
-
Biophysical basis of the phase response curve of subthalamic neurons with generalization to other cell types.J Neurophysiol. 2012 Oct;108(7):1838-55. doi: 10.1152/jn.00054.2012. Epub 2012 Jul 11. J Neurophysiol. 2012. PMID: 22786959 Free PMC article.
References
-
- Abel HJ, Lee JCF, Callaway JC, Foehring RC. Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramidal neurons. J Neurophysiol. 2003;91:324–335. - PubMed
-
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. - PubMed
-
- Baldissera F, Gustafsson B, Parmiggiani F. Saturating summation of the afterhyperpolarization conductance in spinal motoneurones: a mechanism for ‘secondary range’ repetitive firing. Brain Res. 1978;146:69–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

