Exaggerated neointima formation in human C-reactive protein transgenic mice is IgG Fc receptor type I (Fc gamma RI)-dependent
- PMID: 18063701
- PMCID: PMC2189617
- DOI: 10.2353/ajpath.2008.070154
Exaggerated neointima formation in human C-reactive protein transgenic mice is IgG Fc receptor type I (Fc gamma RI)-dependent
Abstract
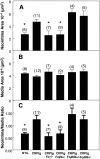
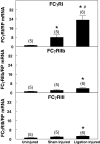
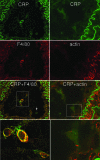
Neointima formation after vascular injury is exaggerated in ovariectomized (OVX) human C-reactive protein transgenic mice (CRPtg) compared to nontransgenic mice (NTG). We tested the hypothesis that this CRP-mediated exacerbation requires IgG Fc receptors (Fc gamma Rs). OVX NTG, CRPtg, and CRPtg lacking Fc gamma RI, Fc gamma RIIb, Fc gamma RIII, or the common gamma chain (FcR gamma) had their common carotid artery ligated. Twenty-eight days later neointimal thickening in CRPtg/Fc gamma RI(-/-) and CRPtg/FcR gamma (-/-) was significantly less than in CRPtg and no worse than in NTG, whereas in CRPtg/Fc gamma RIIb(-/-) and CRPtg/Fc gamma RIII(-/-) neointimal thickness was equal to or greater than in CRPtg. Immunohistochemistry revealed human CRP in the neointima of CRPtg, but little or none was observed in those lacking Fc gamma RI or FcR gamma. Real-time reverse transcriptase-polymerase chain reaction demonstrated that Fc gamma R types I to III were expressed in the CRPtg arteries, with Fc gamma RI expression increasing by threefold after ligation injury. Levels of serum complement (C3), neointimal deposition of complement (C3d), and cellular composition (monocytes, macrophages, lymphocytes) in the neointima did not differ among the different CRPtg genotypes. However, by immunofluorescence a neointimal population of F4/80+CRP+ cells was revealed only in OVX CRPtg. The exaggerated response to vascular injury provoked by CRP in OVX CRPtg depends on Fc gamma RI and probably requires its expression by F4/80+ cells.
Figures

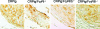

References
-
- van Leeuwen MA, van Rijswijk MH. Acute phase proteins in the monitoring of inflammatory disorders. Baillieres Clin Rheumatol. 1994;8:531–552. - PubMed
-
- Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29:439–493. - PubMed
-
- Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103:2531–2534. - PubMed
-
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. - PubMed
-
- Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–1441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

