Function, structure, and evolution of the RubisCO-like proteins and their RubisCO homologs
- PMID: 18063718
- PMCID: PMC2168653
- DOI: 10.1128/MMBR.00015-07
Function, structure, and evolution of the RubisCO-like proteins and their RubisCO homologs
Abstract
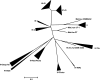
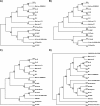
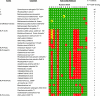
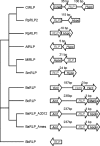
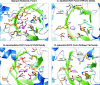
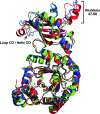
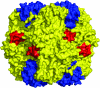
About 30 years have now passed since it was discovered that microbes synthesize RubisCO molecules that differ from the typical plant paradigm. RubisCOs of forms I, II, and III catalyze CO(2) fixation reactions, albeit for potentially different physiological purposes, while the RubisCO-like protein (RLP) (form IV RubisCO) has evolved, thus far at least, to catalyze reactions that are important for sulfur metabolism. RubisCO is the major global CO(2) fixation catalyst, and RLP is a somewhat related protein, exemplified by the fact that some of the latter proteins, along with RubisCO, catalyze similar enolization reactions as a part of their respective catalytic mechanisms. RLP in some organisms catalyzes a key reaction of a methionine salvage pathway, while in green sulfur bacteria, RLP plays a role in oxidative thiosulfate metabolism. In many organisms, the function of RLP is unknown. Indeed, there now appear to be at least six different clades of RLP molecules found in nature. Consideration of the many RubisCO (forms I, II, and III) and RLP (form IV) sequences in the database has subsequently led to a coherent picture of how these proteins may have evolved, with a form III RubisCO arising from the Methanomicrobia as the most likely ultimate source of all RubisCO and RLP lineages. In addition, structure-function analyses of RLP and RubisCO have provided information as to how the active sites of these proteins have evolved for their specific functions.
Figures








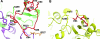
References
-
- Abascal, F., R. Zardoya, and D. Posada. 2005. Prottest: selection of best-fit models of protein evolution. Bioinformatics 21:2104-2105. - PubMed
-
- Alfreider, A., C. Vogt, D. Hoffmann, and W. Babel. 2003. Diversity of ribulose-1,5-bisphosphate carboxylase/oxygenase large-subunit genes from groundwater and aquifer microorganisms. Microb. Ecol. 45:317-328. - PubMed
-
- Anantharaman, V., L. Aravind, and E. V. Koonin. 2003. Emergence of diverse biochemical activities in evolutionarily conserved structural scaffolds of proteins. Curr. Opin. Chem. Biol. 7:12-20. - PubMed
-
- Andersson, I. 1996. Large structures at high resolution: the 1.6 Å crystal structure of spinach ribulose-1,5-bisphosphate carboxylase/oxygenase complexed with 2-carboxyarabinitol bisphosphate. J. Mol. Biol. 259:160-174. - PubMed
-
- Andersson, I., and T. C. Taylor. 2003. Structural framework for catalysis and regulation in ribulose-1,5-bisphosphate carboxylase/oxygenase. Arch. Biochem. Biophys. 414:130-140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

