The autodisplay story, from discovery to biotechnical and biomedical applications
- PMID: 18063719
- PMCID: PMC2168652
- DOI: 10.1128/MMBR.00011-07
The autodisplay story, from discovery to biotechnical and biomedical applications
Abstract
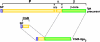
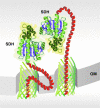
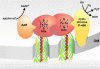
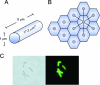
Among the pathways used by gram-negative bacteria for protein secretion, the autotransporter pathway represents a solution of impressive simplicity. Proteins are transported, independent of their nature as recombinant or native passengers, as long as the coding nucleotide sequence is inserted in frame between those of an N-terminal signal peptide and a C-terminal domain, referred to as the beta-barrel of the outer membrane translocation unit. The immunoglobulin A1 (IgA1) protease from Neisseria gonorrhoeae was the first identified member of the autotransporter family of secreted proteins. The IgA1 protease was employed in initial experiments investigating autotransporter-mediated surface display of recombinant proteins and to investigate structural and functional requirements. Various other autotransporter proteins have since been described, and the autodisplay system was developed on the basis of the natural Escherichia coli autotransporter protein AIDA-I (adhesin involved in diffuse adherence). Autodisplay has been used for the surface display of random peptide libraries to successfully screen for novel enzyme inhibitors. The autodisplay system was also used for the surface display of functional enzymes, including esterases, oxidoreductases, and electron transfer proteins. Whole E. coli cells displaying enzymes have been utilized to efficiently synthesize industrially important rare organic compounds with specific chirality. Autodisplay of epitopes on the surface of attenuated Salmonella carriers has also provided a novel way to induce immune protection after oral vaccination. This review summarizes the structural and functional features of the autodisplay system, illustrating its discovery and most recent applications. Autodisplay facilitates the export of more than 100,000 recombinant molecules per single cell and permits the oligomerization of subunits on the cell surface as well as the incorporation of inorganic prosthetic groups after transport of apoproteins onto the bacterial surface without disturbing bacterial integrity or viability. We discuss future biotechnical and biomedical applications in the light of these achievements.
Figures


References
-
- Barbar, E., G. Barany, and C. Woodward. 1996. Unfolded BPTI variants with a single disulfide bond have diminished non-native structure distant from the crosslink. Fold. Des. 1:65-76. - PubMed
-
- Becker, S., H. U. Schmoldt, T. M. Adams, S. Wilhelm, and H. Kolmar. 2004. Ultra-high-throughput screening based on cell-surface display and fluorescence-activated cell sorting for the identification of novel biocatalysts. Curr. Opin. Biotechnol. 15:323-329. - PubMed
-
- Becker, S., S. Theile, N. Heppeler, A. Michalczyk, A. Wentzel, S. Wilhelm, K. E. Jaeger, and H. Kolmar. 2005. A generic system for the Escherichia coli cell-surface display of lipolytic enzymes. FEBS Lett. 579:1177-1182. - PubMed
-
- Benhar, I. 2001. Biotechnological applications of phage and cell display. Biotechnol. Adv. 19:1-33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

