Lipid intermediates in the biosynthesis of bacterial peptidoglycan
- PMID: 18063720
- PMCID: PMC2168651
- DOI: 10.1128/MMBR.00016-07
Lipid intermediates in the biosynthesis of bacterial peptidoglycan
Abstract
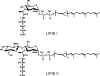
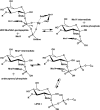
This review is an attempt to bring together and critically evaluate the now-abundant but dispersed data concerning the lipid intermediates of the biosynthesis of bacterial peptidoglycan. Lipid I, lipid II, and their modified forms play a key role not only as the specific link between the intracellular synthesis of the peptidoglycan monomer unit and the extracytoplasmic polymerization reactions but also in the attachment of proteins to the bacterial cell wall and in the mechanisms of action of antibiotics with which they form specific complexes. The survey deals first with their detection, purification, structure, and preparation by chemical and enzymatic methods. The recent important advances in the study of transferases MraY and MurG, responsible for the formation of lipids I and II, are reported. Various modifications undergone by lipids I and II are described, especially those occurring in gram-positive organisms. The following section concerns the cellular location of the lipid intermediates and the translocation of lipid II across the cytoplasmic membrane. The great efforts made since 2000 in the study of the glycosyltransferases catalyzing the glycan chain formation with lipid II or analogues are analyzed in detail. Finally, examples of antibiotics forming complexes with the lipid intermediates are presented.
Figures



References
-
- Anderson, J. S., M. Matsuhashi, M. A. Haskin, and J. L. Strominger. 1967. Biosythesis of the peptidoglycan of bacterial cell walls. II. Phospholipid carriers in the reaction sequence. J. Biol. Chem. 242:3180-3190. - PubMed
-
- Araki, Y., A. Shimada, and E. Ito. 1966. Effect of penicillin on cell wall mucopeptide synthesis in a Escherichia coli particulate system. Biochem. Biophys. Res. Commun. 23:518-525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

