Manipulation of rab GTPase function by intracellular bacterial pathogens
- PMID: 18063721
- PMCID: PMC2168649
- DOI: 10.1128/MMBR.00023-07
Manipulation of rab GTPase function by intracellular bacterial pathogens
Abstract
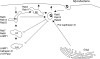
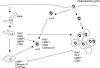
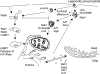
Intracellular bacterial pathogens have evolved highly specialized mechanisms to enter and survive within their eukaryotic hosts. In order to do this, bacterial pathogens need to avoid host cell degradation and obtain nutrients and biosynthetic precursors, as well as evade detection by the host immune system. To create an intracellular niche that is favorable for replication, some intracellular pathogens inhibit the maturation of the phagosome or exit the endocytic pathway by modifying the identity of their phagosome through the exploitation of host cell trafficking pathways. In eukaryotic cells, organelle identity is determined, in part, by the composition of active Rab GTPases on the membranes of each organelle. This review describes our current understanding of how selected bacterial pathogens regulate host trafficking pathways by the selective inclusion or retention of Rab GTPases on membranes of the vacuoles that they occupy in host cells during infection.
Figures



References
-
- Allan, B. B., B. D. Moyer, and W. E. Balch. 2000. Rab1 recruitment of p115 into cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289:444-448. - PubMed
-
- Alvarez-Dominguez, C., A. M. Barbieri, W. Berón, A. Wandinger-Ness, and P. D. Stahl. 1996. Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J. Biol. Chem. 271:13834-13843. - PubMed
-
- Alvarez-Dominguez, C., and P. D. Stahl. 1999. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J. Biol. Chem. 274:11459-11462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

