Escape from suppression: tumor-specific effector cells outcompete regulatory T cells following stem-cell transplantation
- PMID: 18063750
- PMCID: PMC2234051
- DOI: 10.1182/blood-2007-06-096586
Escape from suppression: tumor-specific effector cells outcompete regulatory T cells following stem-cell transplantation
Abstract
Immune reconstitution of autologous hematopoietic stem-cell transplant recipients with the progeny of mature T cells in the graft leads to profound changes in the emerging functional T-cell repertoire. In the steady state, the host is frequently tolerant to tumor antigens, reflecting dominant suppression of naive and effector T cells by regulatory T cells (T(regs)). We examined the relative frequency and function of these 3 components within the tumor-specific T-cell compartment during immune reconstitution. Grafts from tumor-bearing donors exerted a significant antitumor effect in irradiated, syngeneic tumor-bearing recipients. This was associated with dramatic clonal expansion and interferon-gamma (IFNgamma) production by previously tolerant tumor-specific T cells. While donor-derived T(regs) expanded in recipients, they did not inhibit the antigen-driven expansion of effector T cells in the early posttransplantation period. Indeed, the repopulation of tumor-specific effector T cells significantly exceeded that of T(regs), the expansion of which was limited by IL-2 availability. Although the intrinsic suppressive capacity of T(regs) remained intact, their diminished frequency was insufficient to suppress effector cell function. These findings provide an explanation for the reversal of tolerance leading to tumor rejection in transplant recipients and likely contribute to the efficacy of adoptive T-cell therapies in lymphopenic hosts.
Figures





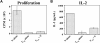
References
-
- Levitsky HI. Augmentation of host immune responses to cancer: overcoming the barrier of tumor antigen-specific T-cell tolerance. Cancer J. 2000;6:S281–290. - PubMed
-
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. - PubMed
-
- Greenberg PD, Cheever MA, Fefer A. Detection of early and delayed antitumor effects following curative adoptive chemoimmunotherapy of established leukemia. Cancer Res. 1980;40:4428–4432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

