Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide
- PMID: 18063812
- PMCID: PMC2869198
- DOI: 10.1161/CIRCRESAHA.107.163253
Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide
Abstract
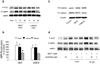
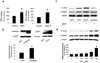
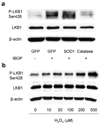
Thromboxane A2 receptor (TPr) stimulation induces cellular hypertrophy in vascular smooth muscle cells (VSMCs); however, regulation of VSMC hypertrophy remains poorly understood. Here we show that TPr stimulation activates AMP-activated kinase (AMPK), which in turn limits TPr-induced protein synthesis in VSMCs. Exposure of cultured VSMCs to either TPr agonists, IBOP and U46619, or exogenous hydrogen peroxide (H2O2) caused time- and dose-dependent AMPK activation, as evidenced by increased phosphorylation of both AMPK-Thr172 and acetyl-coenzyme A carboxylase-Ser79, a downstream enzyme of AMPK, whereas SQ29548, a selective TPr antagonist, significantly attenuated TPr-enhanced AMPK activation. In parallel, both IBOP and U46619 significantly increased the production of reactive oxygen species such as H2O2. Furthermore, adenoviral overexpression of catalase (an H2O2 scavenger) abolished, whereas superoxide dismutase (which catalyzes H2O2 formation) enhanced, IBOP-induced AMPK activation, suggesting that TPr-activated AMPK was mediated by H2O2. Consistently, exposure of VSMCs to either TPr agonists or exogenous H2O2 dose-dependently increased the phosphorylation of LKB1 (at serines 428 and 307), an AMPK kinase, as well as coimmunoprecipitation of AMPK with LKB1. In addition, direct mutagenesis of either Ser428 or Ser307 of LKB1 into alanine, like the kinase-dead LKB1 mutant, abolished both TPr-stimulated AMPK activation and coimmunoprecipitation. Finally, genetic inhibition of AMPK significantly accentuated IBOP-enhanced protein synthesis, whereas adenoviral overexpression of constitutively active AMPK abolished IBOP-enhance protein synthesis in VSMCs. We conclude that TPr stimulation triggers reactive oxygen species-mediated LKB1-dependent AMPK activation, which in return inhibits cellular protein synthesis in VSMCs.
Figures




Similar articles
-
Activation of NAD(P)H oxidases by thromboxane A2 receptor uncouples endothelial nitric oxide synthase.Arterioscler Thromb Vasc Biol. 2011 Jan;31(1):125-32. doi: 10.1161/ATVBAHA.110.207712. Epub 2010 Oct 14. Arterioscler Thromb Vasc Biol. 2011. PMID: 20947827 Free PMC article.
-
Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells.Circulation. 2008 Feb 19;117(7):952-62. doi: 10.1161/CIRCULATIONAHA.107.744490. Epub 2008 Feb 4. Circulation. 2008. PMID: 18250273 Free PMC article.
-
Thromboxane A2 receptor activates a Rho-associated kinase/LKB1/PTEN pathway to attenuate endothelium insulin signaling.J Biol Chem. 2009 Jun 19;284(25):17120-17128. doi: 10.1074/jbc.M109.012583. Epub 2009 Apr 29. J Biol Chem. 2009. Retraction in: J Biol Chem. 2019 Sep 13;294(37):13830. doi: 10.1074/jbc.W119.010660. PMID: 19403525 Free PMC article. Retracted.
-
AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function.Clin Exp Pharmacol Physiol. 2008 May;35(5-6):535-45. doi: 10.1111/j.1440-1681.2007.04851.x. Epub 2007 Dec 26. Clin Exp Pharmacol Physiol. 2008. PMID: 18177481 Free PMC article. Review.
-
Role of AMP-activated protein kinase in the regulation of gene transcription.Biochem Soc Trans. 2002 Apr;30(2):307-11. doi: 10.1042/bst0300307. Biochem Soc Trans. 2002. PMID: 12023870 Review.
Cited by
-
Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo.Am J Pathol. 2013 Mar;182(3):1021-30. doi: 10.1016/j.ajpath.2012.11.022. Epub 2013 Jan 7. Am J Pathol. 2013. PMID: 23306156 Free PMC article.
-
Involvement of vascular peroxidase 1 in angiotensin II-induced vascular smooth muscle cell proliferation.Cardiovasc Res. 2011 Jul 1;91(1):27-36. doi: 10.1093/cvr/cvr042. Epub 2011 Feb 3. Cardiovasc Res. 2011. PMID: 21292788 Free PMC article.
-
Toll-like receptor 4 engagement inhibits adenosine 5'-monophosphate-activated protein kinase activation through a high mobility group box 1 protein-dependent mechanism.Mol Med. 2012 May 9;18(1):659-68. doi: 10.2119/molmed.2011.00401. Mol Med. 2012. PMID: 22396017 Free PMC article.
-
Salidroside exerts protective effects against chronic hypoxia-induced pulmonary arterial hypertension via AMPKα1-dependent pathways.Am J Transl Res. 2016 Jan 15;8(1):12-27. eCollection 2016. Am J Transl Res. 2016. PMID: 27069536 Free PMC article.
-
AMPK, Mitochondrial Function, and Cardiovascular Disease.Int J Mol Sci. 2020 Jul 15;21(14):4987. doi: 10.3390/ijms21144987. Int J Mol Sci. 2020. PMID: 32679729 Free PMC article. Review.
References
-
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. - PubMed
-
- Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van DB, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–168. - PubMed
-
- Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–E10. - PubMed
-
- Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004;36:28–34. - PubMed
-
- Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

