Estrogen determines sex differences in airway responsiveness after allergen exposure
- PMID: 18063836
- PMCID: PMC2335333
- DOI: 10.1165/rcmb.2007-0298OC
Estrogen determines sex differences in airway responsiveness after allergen exposure
Abstract
The female hormone estrogen is an important factor in the regulation of airway function and inflammation, and sex differences in the prevalence of asthma are well described. Using an animal model, we determined how sex differences may underlie the development of altered airway function in response to allergen exposure. We compared sex differences in the development of airway hyperresponsiveness (AHR) after allergen exposure exclusively via the airways. Ovalbumin (OVA) was administered by nebulization on 10 consecutive days in BALB/c mice. After methacholine challenge, significant AHR developed in male mice but not in female mice. Ovariectomized female mice showed significant AHR after 10-day OVA inhalation. ICI182,780, an estrogen antagonist, similarly enhanced airway responsiveness even when administered 1 hour before assay. In contrast, 17beta-estradiol dose-dependently suppressed AHR in male mice. In all cases, airway responsiveness was inhibited by the administration of a neurokinin 1 receptor antagonist. These results demonstrate that sex differences in 10-day OVA-induced AHR are due to endogenous estrogen, which negatively regulates airway responsiveness in female mice. Cumulatively, the results suggest that endogenous estrogen may regulate the neurokinin 1-dependent prejunctional activation of airway smooth muscle in allergen-exposed mice.
Figures
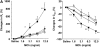
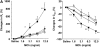
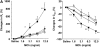


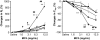


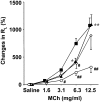
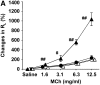



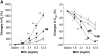
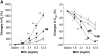

Comment in
-
Sexual tension in the airways: the puzzling duality of estrogen in asthma.Am J Respir Cell Mol Biol. 2008 May;38(5):499-500. doi: 10.1165/rcmb.2008-0002ED. Am J Respir Cell Mol Biol. 2008. PMID: 18417757 No abstract available.
Similar articles
-
Ovalbumin-sensitized mice are good models for airway hyperresponsiveness but not acute physiological responses to allergen inhalation.Clin Exp Allergy. 2008 May;38(5):829-38. doi: 10.1111/j.1365-2222.2007.02884.x. Epub 2007 Dec 7. Clin Exp Allergy. 2008. PMID: 18070158
-
Estrogen replacement therapy prevents airway dysfunction in a murine model of allergen-induced asthma.Lung. 2009 Mar-Apr;187(2):116-27. doi: 10.1007/s00408-008-9129-z. Epub 2008 Dec 13. Lung. 2009. PMID: 19083056
-
Mast cells, Fc epsilon RI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant.J Immunol. 2004 May 15;172(10):6398-406. doi: 10.4049/jimmunol.172.10.6398. J Immunol. 2004. PMID: 15128831
-
Strain-specific phenotypes of airway inflammation and bronchial hyperresponsiveness induced by epicutaneous allergen sensitization in BALB/c and C57BL/6 mice.Int Arch Allergy Immunol. 2010;152 Suppl 1:67-74. doi: 10.1159/000312128. Epub 2010 Jun 4. Int Arch Allergy Immunol. 2010. PMID: 20523066
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
Cited by
-
Sex Differences in Exercise-Induced Bronchoconstriction in Athletes: A Systematic Review and Meta-Analysis.Int J Environ Res Public Health. 2020 Oct 5;17(19):7270. doi: 10.3390/ijerph17197270. Int J Environ Res Public Health. 2020. PMID: 33027929 Free PMC article.
-
Sex steroid signaling: implications for lung diseases.Pharmacol Ther. 2015 Jun;150:94-108. doi: 10.1016/j.pharmthera.2015.01.007. Epub 2015 Jan 14. Pharmacol Ther. 2015. PMID: 25595323 Free PMC article. Review.
-
Perimenstrual Asthma in Adolescents: A Shared Condition in Pediatric and Gynecological Endocrinology.Children (Basel). 2022 Feb 10;9(2):233. doi: 10.3390/children9020233. Children (Basel). 2022. PMID: 35204953 Free PMC article. Review.
-
Arginase 1 deletion in myeloid cells affects the inflammatory response in allergic asthma, but not lung mechanics, in female mice.BMC Pulm Med. 2017 Nov 28;17(1):158. doi: 10.1186/s12890-017-0490-7. BMC Pulm Med. 2017. PMID: 29183288 Free PMC article.
-
Kisspeptins inhibit human airway smooth muscle proliferation.JCI Insight. 2022 May 23;7(10):e152762. doi: 10.1172/jci.insight.152762. JCI Insight. 2022. PMID: 35420998 Free PMC article.
References
-
- Meyer MR, Haas E, Barton M. Sex differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension 2006;47:1019–1026. - PubMed
-
- Most W, van der Wee-Pals L, Ederveen A, Papapoulos S, Lowik C. Ovariectomy and orchidectomy induce a transient increase in the osteoclastogenic potential of bone marrow cells in the mouse. Bone 1997;20:27–30. - PubMed
-
- Gillies GE, Murray HE, Dexter D, McArthur S. Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson's disease. Pharmacol Biochem Behav 2004;78:513–522. - PubMed
-
- Dimitropoulou C, White RE, Ownby DR, Catravas JD. Estrogen reduces carbachol-induced constriction of asthmatic airways by stimulating large-conductance voltage and calcium-dependent potassium channels. Am J Respir Cell Mol Biol 2005;32:239–247. - PubMed
-
- Degano B, Prevost MC, Berger P, Molimard M, Pontier S, Rami J, Escamilla R. Estradiol decreases the acetylcholine-elicited airway reactivity in ovariectomized rats through an increase in epithelial acetylcholinesterase activity. Am J Respir Crit Care Med 2001;164:1849–1854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

